Exploiting lymphatic vessels for immunomodulation: Rationale, opportunities, and challenges
- PMID: 28694027
- PMCID: PMC6026542
- DOI: 10.1016/j.addr.2017.07.005
Exploiting lymphatic vessels for immunomodulation: Rationale, opportunities, and challenges
Abstract
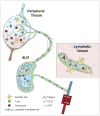
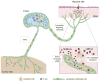
Lymphatic vessels are the primary route of communication from peripheral tissues to the immune system; as such, they represent an important component of local immunity. In addition to their transport functions, new immunomodulatory roles for lymphatic vessels and lymphatic endothelial cells have come to light in recent years, demonstrating that lymphatic vessels help shape immune responses in a variety of ways: promoting tolerance to self-antigens, archiving antigen for later presentation, dampening effector immune responses, and resolving inflammation, among others. In addition to these new biological insights, the growing field of immunoengineering has begun to explore therapeutic approaches to utilize or exploit the lymphatic system for immunotherapy.
Keywords: Cancer; Immune regulation; Immunotherapy; Lymph node targeting; Lymphangiogenesis; Nanotechnology.
Copyright © 2017. Published by Elsevier B.V.
Figures

References
-
- Schmid-Schonbein GW. Microlymphatics and lymph flow. Physiol Rev. 1990;70:987–1028. - PubMed
-
- Wiig H, Swartz MA. Interstitial fluid and lymph formation and transport: physiological regulation and roles in inflammation and cancer. Physiol Rev. 2012;92:1005–1060. - PubMed
-
- Triacca V, Guc E, Kilarski WW, Pisano M, Swartz MA. Transcellular pathways in lymphatic endothelial cells regulate changes in solute transport by fluid stress. Circ Res. 2017;120:1440–1452. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

