PLCζ is the physiological trigger of the Ca2+ oscillations that induce embryogenesis in mammals but conception can occur in its absence
- PMID: 28694258
- PMCID: PMC5592814
- DOI: 10.1242/dev.150227
PLCζ is the physiological trigger of the Ca2+ oscillations that induce embryogenesis in mammals but conception can occur in its absence
Abstract
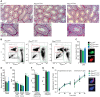
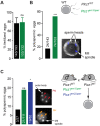
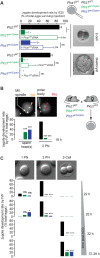
Activation of the egg by the sperm is the first, vital stage of embryogenesis. The sperm protein PLCζ has been proposed as the physiological agent that triggers the Ca2+ oscillations that normally initiate embryogenesis. Consistent with this, recombinant PLCζ induces Ca2+ oscillations in eggs and debilitating mutations in the PLCZ1 gene are associated with infertility in men. However, there has been no evidence that knockout of the gene encoding PLCζ abolishes the ability of sperm to induce Ca2+ oscillations in eggs. Here, we show that sperm derived from Plcz1-/- male mice fail to trigger Ca2+ oscillations in eggs, cause polyspermy and thus demonstrate that PLCζ is the physiological trigger of these Ca2+ oscillations. Remarkably, some eggs fertilized by PLCζ-null sperm can develop, albeit at greatly reduced efficiency, and after a significant time-delay. In addition, Plcz1-/- males are subfertile but not sterile, suggesting that in the absence of PLCζ, spontaneous egg activation can eventually occur via an alternative route. This is the first demonstration that in vivo fertilization without the normal physiological trigger of egg activation can result in offspring. PLCζ-null sperm now make it possible to resolve long-standing questions in fertilization biology, and to test the efficacy and safety of procedures used to treat human infertility.
Keywords: Calcium signalling; Egg; Embryogenesis; Mouse; PLCζ; Sperm.
© 2017. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures



References
-
- Aarabi M., Yu Y., Xu W., Tse M. Y., Pang S. C., Yi Y.-J., Sutovsky P. and Oko R. (2012). The testicular and epididymal expression profile of PLCzeta in mouse and human does not support its role as a sperm-borne oocyte activating factor. PLoS ONE 7, e33496 10.1371/journal.pone.0033496 - DOI - PMC - PubMed
-
- Bernhardt M. L., Lowther K. M., Padilla-Banks E., McDonough C. E., Lee K. N., Evsikov A. V., Uliasz T. F., Chidiac P., Williams C. J. and Mehlmann L. M. (2015). Regulator of G-protein signaling 2 (RGS2) suppresses premature calcium release in mouse eggs. Development 142, 2633-2640. 10.1242/dev.121707 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

