Chronic lung inflammation primes humoral immunity and augments antipneumococcal resistance
- PMID: 28694492
- PMCID: PMC5504016
- DOI: 10.1038/s41598-017-05212-4
Chronic lung inflammation primes humoral immunity and augments antipneumococcal resistance
Abstract
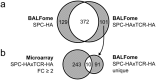
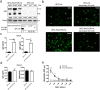
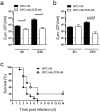
Airway epithelial cells (AECs) display remarkable plasticity in response to infectious stimuli and their functional adaptations are critical for antimicrobial immunity. However, the roles of AECs and humoral mediators to host defense in non-communicable lung inflammation remain elusive. We dissected pulmonary defense against Streptococcus pneumoniae in hosts with pre-existing inflammatory conditions (SPC-HAxTCR-HA mice). Lung tissue transcriptomics and bronchoalveolar lavage fluid (BALF) proteomics revealed an induction of humoral defense mechanisms in inflamed lungs. Accordingly, besides antibacterial proteins and complement components being overrepresented in inflamed lungs, elevated polymeric immunoglobulin receptor (pIgR)-expression in AECs correlated with increased secretory immunoglobulin (SIg) transport. Consequently, opsonization assays revealed augmented pneumococcal coverage by SIgs present in the BALF of SPC-HAxTCR-HA mice, which was associated with enhanced antipneumococcal resistance. These findings emphasize the immunologic potential of AECs as well as their central role in providing antibacterial protection and put forward pIgR as potential target for therapeutic manipulation in infection-prone individuals.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

