Presence of diabetes autoantigens in extracellular vesicles derived from human islets
- PMID: 28694505
- PMCID: PMC5504025
- DOI: 10.1038/s41598-017-04977-y
Presence of diabetes autoantigens in extracellular vesicles derived from human islets
Abstract
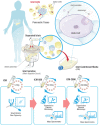
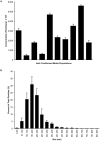
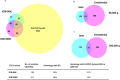
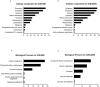
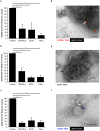
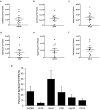
Beta-cell (β-cell) injury is the hallmark of autoimmune diabetes. However, the mechanisms by which autoreactive responses are generated in susceptible individuals are not well understood. Extracellular vesicles (EV) are produced by mammalian cells under normal and stressed physiological states. They are an important part of cellular communication, and may serve a role in antigen processing and presentation. We hypothesized that isolated human islets in culture produce EV that contain diabetes autoantigens (DAA) from these otherwise normal, non-diabetic donors. Here we report the caspase-independent production of EV by human islets in culture, and the characterization of DAA glutamic acid decarboxylase 65 (GAD65) and zinc transporter 8 (ZnT8), as well as the β-cell resident glucose transporter 2 (Glut2), present within the EV.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

