Antivirulent Properties of Underexplored Cinnamomum tamala Essential Oil and Its Synergistic Effects with DNase against Pseudomonas aeruginosa Biofilms - An In Vitro Study
- PMID: 28694794
- PMCID: PMC5483474
- DOI: 10.3389/fmicb.2017.01144
Antivirulent Properties of Underexplored Cinnamomum tamala Essential Oil and Its Synergistic Effects with DNase against Pseudomonas aeruginosa Biofilms - An In Vitro Study
Abstract
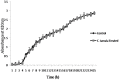
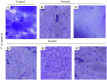
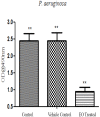
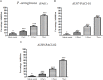
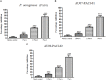
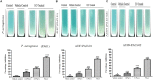
Pseudomonas aeruginosa is a nosocomial pathogen colonizing patients with chronic infectious diseases and has gained resistance to all the known broad spectrum antibiotics available today. The present study showcases the antibiofilm potential of an essential oil (EO) from an underexplored Cinnamomum species namely, C. tamala, against P. aeruginosa biofilms. Furthermore, the synergistic effects of the EO along with a commercially available DNase (DNaseI) and a DNase (MBD) isolated from a marine bacterium were explored for its antibiofilm activity. The results showed that the synergized action has maximum efficacy in inhibiting young and preformed biofilms. The synergized effect of EO and DNaseI showed 70% inhibition against matured biofilms of P. aeruginosa. The EO from C. tamala also showed quorum sensing inhibitory potential as it could inhibit the swarming motility behavior of P. aeruginosa. The synergistic action of EO and DNases offers a novel alternate therapeutic strategy for combating P. aeruginosa biofilm associated infections.
Keywords: Almora; Cinnamomum essential oil; DNase; biofilm; synergism.
Figures






References
-
- Brackman G., Defoirdt T., Miyamoto C., Bossier P., Calenbergh S. V., Nelis H., et al. (2008). Cinnamaldehyde and cinnamaldehyde derivatives reduce virulence in Vibrio spp. By decreasing the DNA-binding activity of the quorum sensing response regulator LuxR. BMC Microbiol. 8:149 10.1186/1471-2180-8-149 - DOI - PMC - PubMed
-
- Clinical and Laboratory Standards Institute (2006). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically. Clinical and Laboratory Standards Institute document M7-A7 7th Edn Wayne, PA: Clinical and Laboratory Standards Institute.
LinkOut - more resources
Full Text Sources
Other Literature Sources

