CD169 Expressing Macrophage, a Key Subset in Mesenteric Lymph Nodes Promotes Mucosal Inflammation in Dextran Sulfate Sodium-Induced Colitis
- PMID: 28694804
- PMCID: PMC5483437
- DOI: 10.3389/fimmu.2017.00669
CD169 Expressing Macrophage, a Key Subset in Mesenteric Lymph Nodes Promotes Mucosal Inflammation in Dextran Sulfate Sodium-Induced Colitis
Abstract
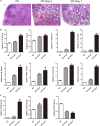
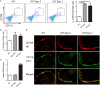
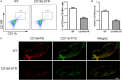
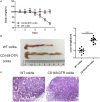
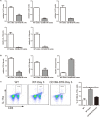
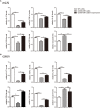
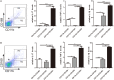
Inflammatory bowel disease (IBD) including Crohn's disease (CD) and ulcerative colitis is a relapsing-remitting illness. Patients with long-standing extensive colitis are easy to develop colorectal cancer (CRC). The increasing incidence of IBD and a substantial increase in the risk of CRC make the necessity to pay more attention on the regulation of inflammation especially by specific macrophages subset. The present study reported that a key subset of sinus macrophage expressing CD169 in mesenteric lymph nodes (mLNs) played an essential role in promoting mucosal inflammation. The results revealed that the subset expressing CD169 in mLNs increased significantly during the dextran sulfate sodium (DSS)-induced colitis. The colitic symptoms were alleviated in CD169-diphtheria toxin receptor (DTR) mice at least partially due to the deletion of CD169+ macrophages in mLNs. In addition, the levels of inflammatory cytokines as well as the percentage of Th17 cells in mLNs from CD169-DTR mice were much lower than those from WT mice with DSS-induced colitis. Further experiment in vitro demonstrated that the supernatant from whole cells of mLNs or colon tissues could promote the production of inflammatory factors by mLN cells or colon tissues from CD169-DTR mice. These results could be explained by the cell sorting result that CD11b+CD169+ macrophages expressed higher level of inflammatory factors directly. All these data indicated that CD169+ sinus macrophage in mLNs played an essential role on regulating mucosal inflammation.
Keywords: CD169+ macrophages; colitis; cytokines; dextran sulfate sodium; mesenteric lymph nodes.
Figures
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

