Physiological and Transcriptional Changes of Three Citrus Rootstock Seedlings under Iron Deficiency
- PMID: 28694816
- PMCID: PMC5483480
- DOI: 10.3389/fpls.2017.01104
Physiological and Transcriptional Changes of Three Citrus Rootstock Seedlings under Iron Deficiency
Abstract
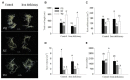
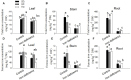
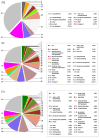
Iron is an essential micronutrient for plants, and plants have evolved adaptive mechanisms to improve iron acquisition from soils. Grafting on iron deficiency-tolerant rootstock is an effective strategy to prevent iron deficiency-chlorosis in fruit-tree crops. To determine the mechanisms underlying iron uptake in iron deficiency, two iron deficiency-tolerant citrus rootstocks, Zhique (ZQ) and Xiangcheng (XC), as well as iron deficiency-sensitive rootstock trifoliate orange (TO) seedlings were studied. Plants were grown in hydroponics system for 100 days, having 50 μM iron (control) and 0 μM iron (iron deficiency) nutrient solution. Under iron deficiency, more obvious visual symptoms of iron chlorosis were observed in the leaves of TO, whereas slight symptoms were observed in ZQ and XC. This was further supported by the lower chlorophyll concentration in the leaves of TO than in leaves of ZQ and XC. Ferrous iron showed no differences among the three citrus rootstock roots, whereas ferrous iron was significantly higher in leaves of ZQ and XC than TO. The specific iron absorption rate and leaf iron proportion were significantly higher in ZQ and XC than in TO, suggesting the iron deficiency tolerance can be explained by increased iron uptake in roots of ZQ and XC, allowed by subsequent translocation to shoots. In transcriptome analysis, 29, 298, and 500 differentially expressed genes (DEGs) in response to iron deficiency were identified in ZQ, XC, and TO, respectively (Fold change ≥ 2 and Probability ≥ 0.8 were used as thresholds to identify DEGs). A Gene Ontology analysis suggested that several genotype-specific biological processes are involved in response to iron deficiency. Genes associated with cell wall biosynthesis, ethylene and abscisic acid signal transduction pathways were involved in iron deficiency responses in citrus rootstocks. The results of this study provide a basis for future analyses of the physiological and molecular mechanisms of the tolerance of different citrus rootstocks to iron deficiency.
Keywords: citrus rootstocks; gene expression regulation; iron concentration; iron deficiency; transcriptome analysis.
Figures



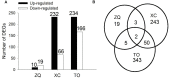

References
-
- An J. C., Liu Y. Z., Yang C. Q., Zhou G. F., Wei Q. J., Peng S. A. (2012). Isolation and expression analysis of CiNIP5, a citrus boron transport gene involved in tolerance to boron deficiency. Sci. Hortic. 142 149–154. 10.1016/j.scienta.2012.05.013 - DOI
-
- Bacaicoa E., García-Mina J. M. (2009). Fe efficiency in different cucumber cultivars: the importance of optimizing the use of foliar Fe. J. Am. Soc. Hortic. Sci. 134 405–416.
-
- Bellaloui N., Brown P. H. (1998). Cultivar differences in boron uptake and distribution in celery (Apium graveolens), tomato (Lycopersicon esculentum) and wheat (Triticum aestivum). Plant Soil 198 153–158. 10.1023/A:1004343031242 - DOI
-
- Carpena-Artes O., Moreno J. J., Lucena J. J., Carpena-Ruiz R. O. (1995). Response to Iron Chlorosis of Different Hydroponically Grown Citrus Varieties. Dordrecht: Springer, 147–151. 10.1007/978-94-011-0503-3_213-3_21 - DOI
-
- Castle W. S., Nunnallee J., Manthey J. A. (2009). Screening citrus rootstocks and related selections in soil and solution culture for tolerance to low-iron stress. Hortscience 44 638–645.
LinkOut - more resources
Full Text Sources
Other Literature Sources

