Strategies toward protecting group-free glycosylation through selective activation of the anomeric center
- PMID: 28694870
- PMCID: PMC5496566
- DOI: 10.3762/bjoc.13.123
Strategies toward protecting group-free glycosylation through selective activation of the anomeric center
Abstract
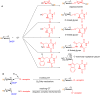
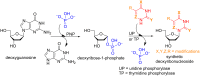
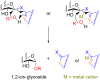
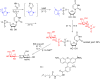
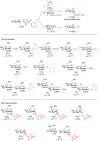
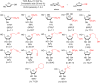
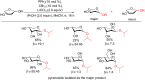
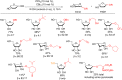
Glycosylation is an immensely important biological process and one that is highly controlled and very efficient in nature. However, in a chemical laboratory the process is much more challenging and usually requires the extensive use of protecting groups to squelch reactivity at undesired reactive moieties. Nonetheless, by taking advantage of the differential reactivity of the anomeric center, a selective activation at this position is possible. As a result, protecting group-free strategies to effect glycosylations are available thanks to the tremendous efforts of many research groups. In this review, we showcase the methods available for the selective activation of the anomeric center on the glycosyl donor and the mechanisms by which the glycosylation reactions take place to illustrate the power these techniques.
Keywords: glycosides; glycosylation; oligosaccharides; protecting groups.
Figures
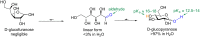




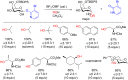

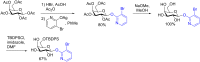

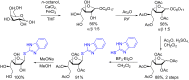
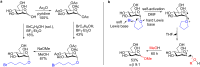





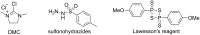
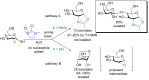
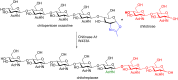
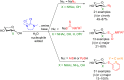











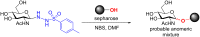

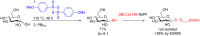
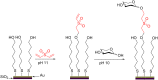


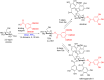
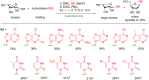


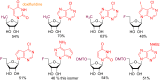

References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
