Molecular engineering of mechanophore activity for stress-responsive polymeric materials
- PMID: 28694949
- PMCID: PMC5485571
- DOI: 10.1039/c4sc01945h
Molecular engineering of mechanophore activity for stress-responsive polymeric materials
Abstract
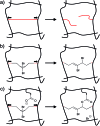
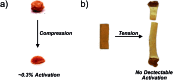
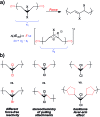
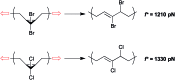
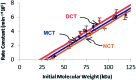
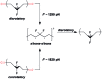
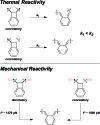
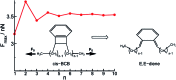
Force reactive functional groups, or mechanophores, have emerged as the basis of a potential strategy for sensing and countering stress-induced material failure. The general utility of this strategy is limited, however, because the levels of mechanophore activation in the bulk are typically low and observed only under large, typically irreversible strains. Strategies that enhance activation are therefore quite useful. Molecular-level design principles by which to engineer enhanced mechanophore activity are reviewed, with an emphasis on quantitative structure-activity studies determined for a family of gem-dihalocyclopropane mechanophores.
Figures



References
-
- Beyer M. K., Clausen-Schaumann H. Chem. Rev. 2005;105:2921. - PubMed
-
- Caruso M. M., Davis D. A., Shen Q., Odom S. A., Sottos N. R., White S. R., Moore J. S. Chem. Rev. 2009;109:5755. - PubMed
-
- Black A. L., Lenhardt J. M., Craig S. L. J. Mater. Chem. 2011;21:1655.
-
- Tashiro K., Wu G., Kobayashi M. Polymer. 1988;29:1768.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

