A mixed methods study to evaluate the feasibility of using the Adolescent Diabetes Needs Assessment Tool App in paediatric diabetes care in preparation for a longitudinal cohort study
- PMID: 28694992
- PMCID: PMC5501574
- DOI: 10.1186/s40814-017-0164-5
A mixed methods study to evaluate the feasibility of using the Adolescent Diabetes Needs Assessment Tool App in paediatric diabetes care in preparation for a longitudinal cohort study
Erratum in
-
Erratum to: Pilot and Feasibility Studies, Vol. 4.Pilot Feasibility Stud. 2017 Oct 24;3:48. doi: 10.1186/s40814-017-0183-2. eCollection 2017. Pilot Feasibility Stud. 2017. PMID: 29123916 Free PMC article.
Abstract
Background: An evaluation study was carried out to determine the feasibility of integrating the Adolescent Diabetes Needs Assessment Tool (ADNAT) App into UK paediatric diabetes care, to ascertain best practice standards and to determine methodological recommendations for a future cohort study.
Methods: A non-randomised, cohort, mixed methods study design was used to ensure equality of access to ADNAT for all participants at three sites in the North West of England. Following UK Medical Research Council guidance, the RE-AIM (reach, effectiveness (potential and perceived), adoption, implementation, maintenance) framework was used to guide study objectives and feasibility outcomes. Patients who completed ADNAT (completers) were compared with those who failed to complete (non-completers). Patients' glycaemic control (HbA1c) was accessed from their clinical data at baseline and at 6 months, alongside their ADNAT scores which were correlated with changes in HbA1c levels. The diabetes teams (respondents) completed a web-based survey and attended focus group interviews.
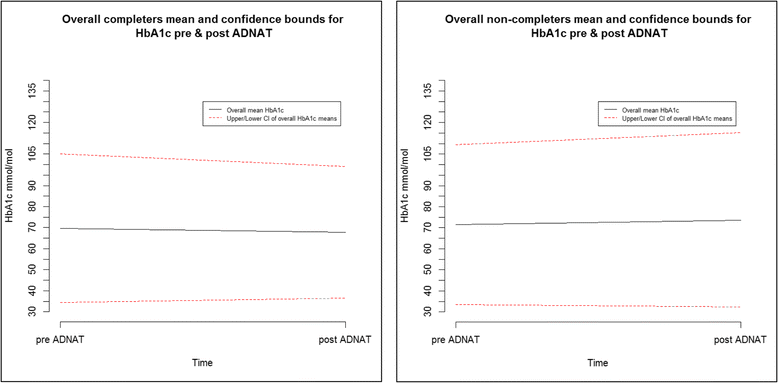
Results: Eighty-nine patients were recruited. Withdrawal rates were low at 4.5% (n = 4). Forty-four patients (49.4%) completed ADNAT, leaving 45 (50.6%) non-completers. There were large baseline differences in HbA1c and variable rates of change at 6 months. After adjusting for baseline HbA1C and site in an analysis of covariance, completers had a lower post-ADNAT mean HbA1C level than non-completers at 6 months (-5.42 mmol/mol, 95% CI -11.48, 0.64). Patients' glycaemic control (HbA1c) at 6 months correlated reasonably well with their ADNAT scores (Spearman's rho = 0.46). Survey and focus group data showed that ADNAT was judged to be an effective clinical tool by the diabetes teams. Value to patients was perceived by the teams to be linked to parental support, age and previous diabetes education. The combined data triangulated. It served to capture different dimensions which were used to define changes to achieve practice standards and methodological recommendations.
Conclusions: The combined data showed that ADNAT has the potential to be a clinically viable tool. It has demonstrated the need for a randomised design that is tailored for a 'hard to reach' adolescent population. A cluster randomised controlled trial that involves sequential but random rollout of ADNAT over multiple time periods may be the most appropriate and is currently being considered for the larger study.
Trial registration: NIHR Children's Clinical Research Network, UKCRN ID 6633.
Keywords: App; Evaluation; Glycaemic control; Needs assessment; Patient education; Questionnaire; Type 1 diabetes.
Figures
References
-
- Pawson R. The science of evaluation: a realist manifesto. Los Angeles, London, New Delhi, Singapore: Sage; 2013.
-
- International Diabetes Federation . IDF diabetes. 7. Brussels, Belgium: International Diabetes Federation; 2015.
-
- NHS Information Centre for Health and Social Care. National Diabetes Paediatric Audit Reports. London: Department of Health. Available at: http://www.rcpch.ac.uk/improving-child-health/quality-improvement-and-cl.... Accessed 15 May 2017.
-
- Diabetes Control and Complications Trial Research Group (1994) Effect of intensive diabetes treatment on the development and progression of long term complications in adolescents with insulin-dependent diabetes mellitus. Journal of Pediatr. 1994;125:177–188. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous