Altered mechanisms of protein synthesis in frontal cortex in Alzheimer disease and a mouse model
- PMID: 28695061
- PMCID: PMC5498849
Altered mechanisms of protein synthesis in frontal cortex in Alzheimer disease and a mouse model
Abstract
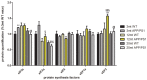
Expression of the nucleolar chaperones nucleolin (NCL) and nucleophosmin (NPM1), upstream binding transcription factor (UBTF), rRNA18S, rRNA28S, and several genes encoding ribosomal proteins (RPs) is decreased in frontal cortex area 8 at advanced stages of Alzheimer's disease (AD). This is accompanied by reduced protein levels of elongation factors eEF1A and eEF2. Changes are more marked in AD cases with rapid course (rpAD), as initiation factor eIF3η is significantly down-regulated and several RP genes up-regulated in rpAD when compared with typical AD. These changes contrast with those seen in APP/PS1 transgenic mice used as a model of AD-like β-amyloidopathy; Ncl mRNA, rRNA18S, rRNA28S and seven out of fifteen assessed RP genes are up-regulated in APP/PS1 mice aged 20 months; only eEF2 protein levels are reduced in transgenic mice. Our findings show marked altered expression of molecules linked to the protein synthesis machinery from the nucleolus to the ribosome in frontal cortex at terminal stages of AD which differs from that seen in APP/PS1 transgenic mice, thus further suggesting that molecular signals in mouse models do not apply to real human disease counterparts.
Keywords: APP/PS1 transgenic mouse; Alzheimer’s disease; nucleolar chaperones; protein synthesis; rRNAs; transcription; translation.
Conflict of interest statement
None.
Figures


References
-
- da Silva AM, Payão SL, Borsatto B, Bertolucci PH, Smith MA. Quantitative evaluation of rRNA in Alzheimer’s disease. Mech Ageing Dev. 2000;120:57–64. - PubMed
-
- Dönmez-Altuntas H, Akalain H, Karaman Y, Demirtas H, Imamoglu N, Ozkul Y. Evaluation of the nucleolar organizer regions in Alzheimer’s disease. Gerontology. 2005;51:297–301. - PubMed
-
- Sajdel-Sulkowska EM, Marotta CA. Alzheimer’s disease brain: alterations in RNA levels and in a ribonuclease-inhibitor complex. Science. 1984;225:947–949. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
