Megalencephalic leukoencephalopathy with cysts: the Glialcam-null mouse model
- PMID: 28695146
- PMCID: PMC5497535
- DOI: 10.1002/acn3.405
Megalencephalic leukoencephalopathy with cysts: the Glialcam-null mouse model
Abstract
Objective: Megalencephalic leukoencephalopathy with cysts (MLC) is a genetic infantile-onset disease characterized by macrocephaly and white matter edema due to loss of MLC1 function. Recessive mutations in either MLC1 or GLIALCAM cause the disease. MLC1 is involved in astrocytic volume regulation; GlialCAM ensures the correct membrane localization of MLC1. Their exact role in brain ion-water homeostasis is only partly defined. We characterized Glialcam-null mice for further studies.
Methods: We investigated the consequences of loss of GlialCAM in Glialcam-null mice and compared GlialCAM developmental expression in mice and men.
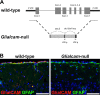
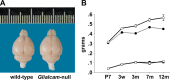
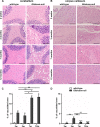
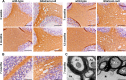
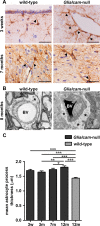
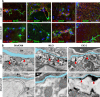
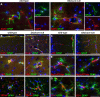
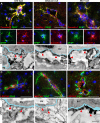
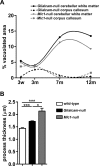
Results: Glialcam-null mice had early-onset megalencephaly and increased brain water content. From 3 weeks, astrocytes were abnormal with swollen processes abutting blood vessels. Concomitantly, progressive white matter vacuolization developed due to intramyelinic edema. Glialcam-null astrocytes showed abolished expression of MLC1, reduced expression of the chloride channel ClC-2 and increased expression and redistribution of the water channel aquaporin4. Expression of other MLC1-interacting proteins and the volume regulated anion channel LRRC8A was unchanged. In mice, GlialCAM expression increased until 3 weeks and then stabilized. In humans, GlialCAM expression was highest in the first 3 years to then decrease and stabilize from approximately 5 years.
Interpretation: Glialcam-null mice replicate the early stages of the human disease with early-onset intramyelinic edema. The earliest change is astrocytic swelling, further substantiating that a defect in astrocytic volume regulation is the primary cellular defect in MLC. GlialCAM expression affects expression of MLC1, ClC-2 and aquaporin4, indicating that abnormal interplay between these proteins is a disease mechanism in megalencephalic leukoencephalopathy with cysts.
Figures

References
-
- van der Knaap MS, Valk J, Barth PG, et al. Leukoencephalopathy with swelling in children and adolescents: MRI patterns and differential diagnosis. Neuroradiology 1995;37:679–686. - PubMed
-
- Singhal BS, Gursahani RD, Udani VP, Biniwale AA. Megalencephalic leukodystrophy in an Asian Indian ethnic group. Pediatr Neurol 1996;14:291–296. - PubMed
-
- van der Voorn JP, Pouwels PJ, Hart AA, et al. Childhood white matter disorders: quantitative MR imaging and spectroscopy. Radiology 2006;241:510–517. - PubMed
-
- Kocaman G, Eryigit G, Abbink TE, et al. An unusually mild presentation of megalencephalic leukoencephalopathy with subcortical cysts. Clin Neurol Neurosurg 2013;115:1564–1566. - PubMed
-
- van der Knaap MS, Barth PG, Vrensen GF, Valk J. Histopathology of an infantile‐onset spongiform leukoencephalopathy with a discrepantly mild clinical course. Acta Neuropathol 1996;92:206–212. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

