Intranasal oxytocin treatment for social deficits and biomarkers of response in children with autism
- PMID: 28696286
- PMCID: PMC5544319
- DOI: 10.1073/pnas.1705521114
Intranasal oxytocin treatment for social deficits and biomarkers of response in children with autism
Abstract
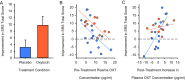
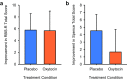
Autism spectrum disorder (ASD) is characterized by core social deficits. Prognosis is poor, in part, because existing medications target only associated ASD features. Emerging evidence suggests that the neuropeptide oxytocin (OXT) may be a blood-based biomarker of social functioning and a possible treatment for ASD. However, prior OXT treatment trials have produced equivocal results, perhaps because of variability in patients' underlying neuropeptide biology, but this hypothesis has not been tested. Using a double-blind, randomized, placebo-controlled, parallel design, we tested the efficacy and tolerability of 4-wk intranasal OXT treatment (24 International Units, twice daily) in 32 children with ASD, aged 6-12 y. When pretreatment neuropeptide measures were included in the statistical model, OXT compared with placebo treatment significantly enhanced social abilities in children with ASD [as measured by the trial's primary outcome measure, the Social Responsiveness Scale (SRS)]. Importantly, pretreatment blood OXT concentrations also predicted treatment response, such that individuals with the lowest pretreatment OXT concentrations showed the greatest social improvement. OXT was well tolerated, and its effects were specific to social functioning, with no observed decrease in repetitive behaviors or anxiety. Finally, as with many trials, some placebo-treated participants showed improvement on the SRS. This enhanced social functioning was mirrored by a posttreatment increase in their blood OXT concentrations, suggesting that increased endogenous OXT secretion may underlie this improvement. These findings indicate that OXT treatment enhances social abilities in children with ASD and that individuals with pretreatment OXT signaling deficits may stand to benefit the most from OXT treatment.
Keywords: autism; biomarkers; clinical trial; oxytocin; social functioning.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders: Diagnostic Criteria for Autistic Disorder. 5th Ed Am Psychiatr Assoc; Washington, DC: 2013.
-
- Developmental Disabilities Monitoring Network Surveillance Year 2010 Principal Investigators Centers for Disease Control and Prevention (CDC) Prevalence of autism spectrum disorder among children aged 8 years–Autism and developmental disabilities monitoring network, 11 sites, United States, 2010. MMWR Surveill Summ. 2014;63:1–21. - PubMed
-
- Buescher AV, Cidav Z, Knapp M, Mandell DS. Costs of autism spectrum disorders in the United Kingdom and the United States. JAMA Pediatr. 2014;168:721–728. - PubMed
-
- Landgraf R, Neumann ID. Vasopressin and oxytocin release within the brain: A dynamic concept of multiple and variable modes of neuropeptide communication. Front Neuroendocrinol. 2004;25:150–176. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

