TRPM7 senses oxidative stress to release Zn2+ from unique intracellular vesicles
- PMID: 28696294
- PMCID: PMC5544332
- DOI: 10.1073/pnas.1707380114
TRPM7 senses oxidative stress to release Zn2+ from unique intracellular vesicles
Abstract
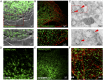
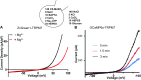
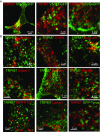
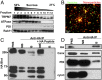
TRPM7 (transient receptor potential cation channel subfamily M member 7) regulates gene expression and stress-induced cytotoxicity and is required in early embryogenesis through organ development. Here, we show that the majority of TRPM7 is localized in abundant intracellular vesicles. These vesicles (M7Vs) are distinct from endosomes, lysosomes, and other familiar vesicles or organelles. M7Vs accumulate Zn2+ in a glutathione-enriched, reduced lumen when cytosolic Zn2+ concentrations are elevated. Treatments that increase reactive oxygen species (ROS) trigger TRPM7-dependent Zn2+ release from the vesicles, whereas reduced glutathione prevents TRPM7-dependent cytosolic Zn2+ influx. These observations strongly support the notion that ROS-mediated TRPM7 activation releases Zn2+ from intracellular vesicles after Zn2+ overload. Like the endoplasmic reticulum, these vesicles are a distributed system for divalent cation uptake and release, but in this case the primary divalent ion is Zn2+ rather than Ca2.
Keywords: TRPM7; vesicles; zinc.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


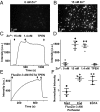
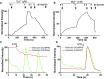
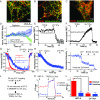
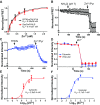

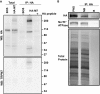
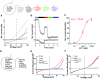

References
-
- Nadler MJ, et al. LTRPC7 is a Mg.ATP-regulated divalent cation channel required for cell viability. Nature. 2001;411:590–595. - PubMed
-
- Runnels LW, Yue L, Clapham DE. TRP-PLIK, a bifunctional protein with kinase and ion channel activities. Science. 2001;291:1043–1047. - PubMed
-
- Aarts M, et al. A key role for TRPM7 channels in anoxic neuronal death. Cell. 2003;115:863–877. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

