Continuous immunotypes describe human immune variation and predict diverse responses
- PMID: 28696306
- PMCID: PMC5544312
- DOI: 10.1073/pnas.1705065114
Continuous immunotypes describe human immune variation and predict diverse responses
Abstract
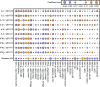
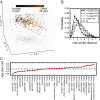
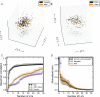
The immune system consists of many specialized cell populations that communicate with each other to achieve systemic immune responses. Our analyses of various measured immune cell population frequencies in healthy humans and their responses to diverse stimuli show that human immune variation is continuous in nature, rather than characterized by discrete groups of similar individuals. We show that the same three key combinations of immune cell population frequencies can define an individual's immunotype and predict a diverse set of functional responses to cytokine stimulation. We find that, even though interindividual variations in specific cell population frequencies can be large, unrelated individuals of younger age have more homogeneous immunotypes than older individuals. Across age groups, cytomegalovirus seropositive individuals displayed immunotypes characteristic of older individuals. The conceptual framework for defining immunotypes suggested by our results could guide the development of better therapies that appropriately modulate collective immunotypes, rather than individual immune components.
Keywords: aging; human immune variation; immune cell composition; systems immunology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
-
- Bandura DR, et al. Mass cytometry: Technique for real time single cell multitarget immunoassay based on inductively coupled plasma time-of-flight mass spectrometry. Anal Chem. 2009;81:6813–6822. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

