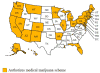
Mapping medical marijuana: state laws regulating patients, product safety, supply chains and dispensaries, 2017
- PMID: 28696583
- PMCID: PMC5725759
- DOI: 10.1111/add.13910
Mapping medical marijuana: state laws regulating patients, product safety, supply chains and dispensaries, 2017
Abstract
Aims: (1) To describe open source legal data sets, created for research use, that capture the key provisions of US state medical marijuana laws. The data document how state lawmakers have regulated a medicine that remains, under federal law, a Schedule I illegal drug with no legitimate medical use. (2) To demonstrate the variability that exists across states in rules governing patient access, product safety and dispensary practice.
Methods: Two legal researchers collected and coded state laws governing marijuana patients, product safety and dispensaries in effect on 1 February 2017, creating three empirical legal data sets. We used summary tables to identify the variation in specific statutory provisions specified in each state's medical marijuana law as it existed on 1 February 2017. We compared aspects of these laws to the traditional Federal approach to regulating medicine. Full data sets, codebooks and protocols are available through the Prescription Drug Abuse Policy System (http://www.pdaps.org/; Archived at http://www.webcitation.org/6qv5CZNaZ on 2 June 2017).
Results: Twenty-eight states (including the District of Columbia) have authorized medical marijuana. Twenty-seven specify qualifying diseases, which differ across states. All states protect patient privacy; only 14 protect patients against discrimination. Eighteen states have mandatory product safety testing before any sale. While the majority have package/label regulations, states have a wide range of specific requirements. Most regulate dispensaries (25 states), with considerable variation in specific provisions such as permitted product supply sources number of dispensaries per state and restricting proximity to various types of location.
Conclusions: The federal ban in the United States on marijuana has resulted in a patchwork of regulatory strategies that are not uniformly consistent with the approach usually taken by the Federal government and whose effectiveness remains unknown.
Keywords: Drug policy; legal epidemiology; marijuana law; medical cannabis; policy surveillance; regulation.
© 2017 Society for the Study of Addiction.
Conflict of interest statement
Declarations of competing interests: None
Figures
References
-
- Vitiello M. Proposition 215: De facto legalization of pot and the shortcomings of direct democracy. U Mich JL Reform. 1997–1998;31(3):707–76.
-
- Drug Enforcement Administration. Drug Scheduling. United States Drug Enforcement Administration; 2016. [cited 2016 June 1]; Available from: http://www.dea.gov/druginfo/ds.shtml; http://www.webcitation.org/6qv4MVD5s.
-
- Cole JM. Memorandum. Washington, D.C: United States Department of Justice; 2013. Aug 29, Guidance Regarding Marijuana Enforcement. Report No.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical