The presence and severity of nonalcoholic steatohepatitis is associated with specific changes in circulating bile acids
- PMID: 28696585
- PMCID: PMC5764808
- DOI: 10.1002/hep.29359
The presence and severity of nonalcoholic steatohepatitis is associated with specific changes in circulating bile acids
Abstract
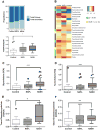
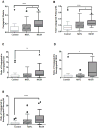
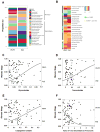
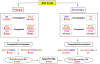
The histologic spectrum of nonalcoholic fatty liver disease (NAFLD) includes fatty liver (NAFL) and steatohepatitis (NASH), which can progress to cirrhosis in up to 20% of NASH patients. Bile acids (BA) are linked to the pathogenesis and therapy of NASH. We (1) characterized the plasma BA profile in biopsy-proven NAFL and NASH and compared to controls and (2) related the plasma BA profile to liver histologic features, disease activity, and fibrosis. Liquid chromatography/mass spectrometry quantified BAs. Descriptive statistics, paired and multiple group comparisons, and regression analyses were performed. Of 86 patients (24 controls, 25 NAFL, and 37 NASH; mean age 51.8 years and body mass index 31.9 kg/m2 ), 66% were women. Increased total primary BAs and decreased secondary BAs (both P < 0.05) characterized NASH. Total conjugated primary BAs were significantly higher in NASH versus NAFL (P = 0.047) and versus controls (P < 0.0001). NASH had higher conjugated to unconjugated chenodeoxycholate (P = 0.04), cholate (P = 0.0004), and total primary BAs (P < 0.0001). The total cholate to chenodeoxycholate ratio was significantly higher in NAFLD without (P = 0.005) and with (P = 0.02) diabetes. Increased key BAs were associated with higher grades of steatosis (taurocholate), lobular (glycocholate) and portal inflammation (taurolithocholate), and hepatocyte ballooning (taurocholate). Conjugated cholate and taurocholate directly and secondary to primary BA ratio inversely correlated to NAFLD activity score. A higher ratio of total secondary to primary BA decreased (odds ratio, 0.57; P = 0.004) and higher conjugated cholate increased the likelihood of significant fibrosis (F≥2) (P = 0.007). Conclusion: NAFLD is associated with significantly altered circulating BA composition, likely unaffected by type 2 diabetes, and correlated with histological features of NASH; these observations provide the foundation for future hypothesis-driven studies of specific effects of BAs on specific aspects of NASH. (Hepatology 2018;67:534-548).
© 2017 by the American Association for the Study of Liver Diseases.
Conflict of interest statement
None: Drs. Puri, Min, Zhou, Hylemon, Santhekadur, Cazanave, Kumar & Ms. Boyett
*Dr. Andrew Joyce: No relevant conflicts. He is Chief Scientific Officer and has an ownership interest in Venebio, based in Richmond
*Faridoddin Mirshahi: No relevant conflicts. Ownership interest in Sanyal Biotechnologies
*Dr. Velimir Luketic: No relevant conflicts. Received grant support from Intercept, Genfit.
*Dr. Mohammed Siddiqui: No relevant conflicts. He has received grant support from Nusirt, Immuron and Taiwan J Pharmaceuticals.
*Dr. Rohit Kohli: No relevant conflicts. He has received grant support/speaker fees from Intercept, Galectin, Raptor, and Shire.
*Dr. Arun Sanyal: None for this project. Dr. Sanyal is President of Sanyal Biotechnology and has stock options in Genfit. He has served as a consultant to AbbVie, Astra Zeneca, Nitto Denko, Nimbus, Salix, Tobira, Takeda, Fibrogen, Lilly, Zafgen, Novartis, Pfizer, Immuron, Exhalenz and Genfit. He has been an unpaid consultant to Intercept, Echosens, Immuron, Amarin, Ardelyx, Fractyl, Syntlogic, Nordic Bioscience and Bristol Myers Squibb. His institution has received grant support from Gilead, Salix, Tobira, Intercept, Merck, Astra Zeneca and Novartis.
Figures




Comment in
-
Bile acids in nonalcoholic steatohepatitis: Pathophysiological driving force or innocent bystanders?Hepatology. 2018 Feb;67(2):464-466. doi: 10.1002/hep.29543. Epub 2017 Dec 23. Hepatology. 2018. PMID: 28926117 No abstract available.
-
Plasma Bile Acid Concentrations in Humans: Suggestions for Presentation in Tabular Form.Hepatology. 2018 Aug;68(2):787. doi: 10.1002/hep.29909. Hepatology. 2018. PMID: 29604219 No abstract available.
References
-
- Rinella ME. Nonalcoholic fatty liver disease: a systematic review. JAMA. 2015;313:2263–2273. - PubMed
-
- Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413–1419. - PubMed
-
- Wong RJ, Aguilar M, Cheung R, Perumpail RB, Harrison SA, Younossi ZM, Ahmed A. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology. 2015;148:547–555. - PubMed
-
- Ridlon JM, Kang DJ, Hylemon PB. Bile salt biotransformations by human intestinal bacteria. J Lipid Res. 2006;47:241–259. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

