Postoperative Adjuvant Therapy for Resectable Pancreatic Cancer With Gemcitabine and Adoptive Immunotherapy
- PMID: 28697053
- PMCID: PMC5555975
- DOI: 10.1097/MPA.0000000000000880
Postoperative Adjuvant Therapy for Resectable Pancreatic Cancer With Gemcitabine and Adoptive Immunotherapy
Abstract
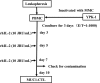
Objectives: We previously described adoptive immunotherapy (AIT) with cytotoxic T lymphocytes (CTLs) stimulated by the mucin 1 (MUC1)-expressing human pancreatic cancer cell line YPK-1 (MUC1-CTLs) and demonstrated that MUC1-CTLs might prevent liver metastasis. In the present study, we combined gemcitabine (GEM) and AIT for the treatment of pancreatic cancer.
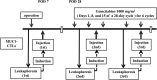
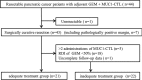
Methods: A total of 43 patients who underwent radical pancreatectomy received treatment with MUC1-CTLs and GEM. After surgery, MUC1-CTLs were induced and administered intravenously 3 times, and GEM administered according to the standard regimen for 6 months. The patients whose relative dose intensity of GEM was 50% or more and who received 2 or more MUC1-CTL treatments were used as the adequate treatment group (n = 21).
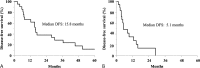
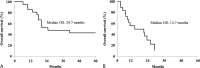
Results: In the adequate treatment group, disease-free survival was 15.8 months, and overall survival was 24.7 months. Liver metastasis was found only in 7 patients (33%), and local recurrence occurred in 4 patients (19%). The independent prognostic factor of long-term disease-free survival on multivariate analysis was the average number of CTLs administered (P = 0.0133).
Conclusions: The combination therapy with AIT and GEM prevented liver metastasis and local recurrence. Moreover, the disease free-survival was improved in patients who received sufficient CTLs.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Sener SF, Fremgen A, Menck HR, et al. Pancreatic cancer: a report of treatment and survival trends for 100,313 patients diagnosed from 1985–1995, using the National Cancer Database. J Am Coll Surg. 1999;189:1–7. - PubMed
-
- Oettle H, Post S, Neuhaus P, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA. 2007;297:267–277. - PubMed
-
- Gendler SJ, Lancaster CA, Taylor-Papadimitriou J, et al. Molecular cloning and expression of human tumor-associated polymorphic epithelial mucin. J Biol Chem. 1990;265:15286–15293. - PubMed
-
- Masaki Y, Oka M, Ogura Y, et al. Sialylated MUC1 mucin expression in normal pancreas, benign pancreatic lesions, and pancreatic ductal adenocarcinoma. Hepatogastroenterology. 1999;46:2240–2245. - PubMed
-
- Kawaoka T, Oka M, Takashima M, et al. Adoptive immunotherapy for pancreatic cancer: cytotoxic T lymphocytes stimulated by the MUC1-expressing human pancreatic cancer cell line YPK-1. Oncol Rep. 2008;20:155–163. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

