Association of Positive Airway Pressure With Cardiovascular Events and Death in Adults With Sleep Apnea: A Systematic Review and Meta-analysis
- PMID: 28697252
- PMCID: PMC5541330
- DOI: 10.1001/jama.2017.7967
Association of Positive Airway Pressure With Cardiovascular Events and Death in Adults With Sleep Apnea: A Systematic Review and Meta-analysis
Abstract
Importance: Sleep apnea (obstructive and central) is associated with adverse cardiovascular risk factors and increased risks of cardiovascular disease. Positive airway pressure (PAP) provides symptomatic relief, whether delivered continuously (CPAP) or as adaptive servo-ventilation (ASV), but the associations with cardiovascular outcomes and death are unclear.
Objective: To assess the association of PAP vs control with cardiovascular events and death in patients with sleep apnea.
Data sources and study selection: MEDLINE, EMBASE, and the Cochrane Library were systematically searched from inception date to March 2017 for randomized clinical trials that included reporting of major adverse cardiovascular events or deaths.
Data extraction and synthesis: Two authors independently extracted data using standardized forms. Summary relative risks (RRs), risk differences (RDs) and 95% CIs were obtained using random-effects meta-analysis.
Main outcomes and measures: The main outcomes were a composite of acute coronary syndrome (ACS) events, stroke, or vascular death (major adverse cardiovascular events); cause-specific vascular events; and death.
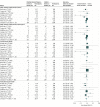
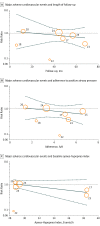
Results: The analyses included data from 10 trials (9 CPAP; 1 ASV) of patients with sleep apnea (N = 7266; mean age, 60.9 [range, 51.5 to 71.1] years; 5847 [80.5%] men; mean [SD] body mass index, 30.0 [5.2]. Among 356 major adverse cardiovascular events and 613 deaths recorded, there was no significant association of PAP with major adverse cardiovascular events (RR, 0.77 [95% CI, 0.53 to 1.13]; P = .19 and RD, -0.01 [95% CI, -0.03 to 0.01]; P = .23), cardiovascular death (RR, 1.15 [95% CI, 0.88 to 1.50]; P = .30 and RD -0.00 [95% CI, -0.02 to 0.02]; P = .87), or all-cause death (RR, 1.13 [95% CI, 0.99 to 1.29]; P = .08 and RD, 0.00 [95% CI, -0.01 to 0.01]; P = .51). The same was true for ACS, stroke, and heart failure. There was no evidence of different associations for CPAP vs ASV (all P value homogeneity >.24), and meta-regressions identified no associations of PAP with outcomes for different levels of apnea severity, follow-up duration, or adherence to PAP (all P values > .13).
Conclusions and relevance: The use of PAP, compared with no treatment or sham, was not associated with reduced risks of cardiovascular outcomes or death for patients with sleep apnea. Although there are other benefits of treatment with PAP for sleep apnea, these findings do not support treatment with PAP with a goal of prevention of these outcomes.
Conflict of interest statement
Figures


Comment in
-
Does Obstructive Sleep Apnea Treatment Reduce Cardiovascular Risk?: It Is Far Too Soon to Say.JAMA. 2017 Jul 11;318(2):128-130. doi: 10.1001/jama.2017.7966. JAMA. 2017. PMID: 28697240 No abstract available.
-
A meta-analysis of positive airway pressure treatment for cardiovascular prevention: why mix apples and pears?Evid Based Med. 2017 Dec;22(6):218-219. doi: 10.1136/ebmed-2017-110835. Epub 2017 Nov 18. Evid Based Med. 2017. PMID: 29151025 Free PMC article. No abstract available.
-
PAP and Cardiovascular Events in Adults With Sleep Apnea: Is PAP Useful?J Clin Sleep Med. 2017 Dec 15;13(12):1487-1489. doi: 10.5664/jcsm.6858. J Clin Sleep Med. 2017. PMID: 29151429 Free PMC article. No abstract available.
-
Review: In adults with sleep apnea, positive airway pressure does not reduce cardiovascular events or death.Ann Intern Med. 2017 Nov 21;167(10):JC54. doi: 10.7326/ACPJC-2017-167-10-054. Ann Intern Med. 2017. PMID: 29159380 No abstract available.
-
Outcomes of Positive Airway Pressure for Sleep Apnea.JAMA. 2017 Nov 28;318(20):2042. doi: 10.1001/jama.2017.16287. JAMA. 2017. PMID: 29183060 No abstract available.
-
Outcomes of Positive Airway Pressure for Sleep Apnea.JAMA. 2017 Nov 28;318(20):2042-2043. doi: 10.1001/jama.2017.16291. JAMA. 2017. PMID: 29183061 No abstract available.
-
Adherence to CPAP May Make a Difference for Cardiovascular Outcomes in Patients with OSA.Am Fam Physician. 2018 Sep 15;98(6):341-342. Am Fam Physician. 2018. PMID: 30215924 No abstract available.
References
-
- Lavie L. Oxidative stress in obstructive sleep apnea and intermittent hypoxia—revisited—the bad ugly and good. Sleep Med Rev. 2015;20:27-45. - PubMed
-
- Phillips CL, McEwen BJ, Morel-Kopp MC, et al. Effects of continuous positive airway pressure on coagulability in obstructive sleep apnoea. Thorax. 2012;67(7):639-644. - PubMed
-
- Somers VK, White DP, Amin R, et al. Sleep apnea and cardiovascular disease: an American Heart Association/American College of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing. J Am Coll Cardiol. 2008;52(8):686-717. - PubMed
-
- Buchanan P, Grunstein R. Positive airway pressure treatment for obstructive sleep apnea-hypopnea syndrome In: Kryger MH, Roth T, Dement WC In: Principles and Practice of Sleep Medicine. 5th ed Philadelphia, PA: Elsevier Saunders; 2011:1233-1249
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

