Transport of the alpha subunit of the voltage gated L-type calcium channel through the sarcoplasmic reticulum occurs prior to localization to triads and requires the beta subunit but not Stac3 in skeletal muscles
- PMID: 28697281
- PMCID: PMC5569907
- DOI: 10.1111/tra.12502
Transport of the alpha subunit of the voltage gated L-type calcium channel through the sarcoplasmic reticulum occurs prior to localization to triads and requires the beta subunit but not Stac3 in skeletal muscles
Abstract
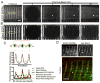
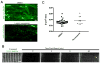
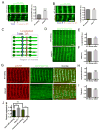
Contraction of skeletal muscle is initiated by excitation-contraction (EC) coupling during which membrane voltage is transduced to intracellular Ca2+ release. EC coupling requires L-type voltage gated Ca2+ channels (the dihydropyridine receptor or DHPR) located at triads, which are junctions between the transverse (T) tubule and sarcoplasmic reticulum (SR) membranes, that sense membrane depolarization in the T tubule membrane. Reduced EC coupling is associated with ageing, and disruptions of EC coupling result in congenital myopathies for which there are few therapies. The precise localization of DHPRs to triads is critical for EC coupling, yet trafficking of the DHPR to triads is not well understood. Using dynamic imaging of zebrafish muscle fibers, we find that DHPR is transported along the longitudinal SR in a microtubule-independent mechanism. Furthermore, transport of DHPR in the SR membrane is differentially affected in null mutants of Stac3 or DHPRβ, two essential components of EC coupling. These findings reveal previously unappreciated features of DHPR motility within the SR prior to assembly at triads.
Keywords: DHPR; EC coupling; Stac3; calcium channel; skeletal muscle; trafficking; zebrafish.
© 2017 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
Conflict of interest statement
Competing interests
The authors have no competing interests associated with the research reported in this study.
Figures




References
-
- Schneider MF, Chandler WK. Voltage dependent charge movement of skeletal muscle: a possible step in excitation-contraction coupling. Nature. 1973;242(5395):244–246. - PubMed
-
- Rios E, Brum G. Involvement of dihydropyridine receptors in excitation-contraction coupling in skeletal muscle. Nature. 1987;325(6106):717–720. - PubMed
-
- Bannister RA. Bridging the myoplasmic gap II: more recent advances in skeletal muscle excitation-contraction coupling. The Journal of experimental biology. 2016;219(Pt 2):175–182. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

