Choose and Use Your Chemical Probe Wisely to Explore Cancer Biology
- PMID: 28697345
- PMCID: PMC5511331
- DOI: 10.1016/j.ccell.2017.06.005
Choose and Use Your Chemical Probe Wisely to Explore Cancer Biology
Erratum in
-
Choose and Use Your Chemical Probe Wisely to Explore Cancer Biology.Cancer Cell. 2017 Aug 14;32(2):268-270. doi: 10.1016/j.ccell.2017.07.010. Cancer Cell. 2017. PMID: 28810148 Free PMC article. No abstract available.
Abstract
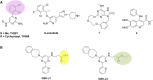
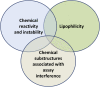
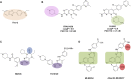
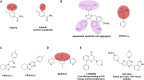
Small-molecule chemical probes or tools have become progressively more important in recent years as valuable reagents to investigate fundamental biological mechanisms and processes causing disease, including cancer. Chemical probes have also achieved greater prominence alongside complementary biological reagents for target validation in drug discovery. However, there is evidence of widespread continuing misuse and promulgation of poor-quality and insufficiently selective chemical probes, perpetuating a worrisome and misleading pollution of the scientific literature. We discuss current challenges with the selection and use of chemical probes, and suggest how biologists can and should be more discriminating in the probes they employ.
Keywords: PAINS; Pan-Assay Interference Compounds; biochemical selectivity; chemical probe; chemical reactivity; chemical tool; ligand promiscuity; lipophilicity; pharmacological audit trail; pharmacophore crossing; target validation.
Crown Copyright © 2017. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- American Association for Cancer Research. (2015). Proceedings: AACR 106th Annual Meeting 2015. Philadelphia. Cancer Res. 75, 15 Supplement.
-
- American Association for Cancer Research. (2017). CICR: general resources. Resources for understanding the proper use of chemical tools in cancer research. http://www.aacr.org/MEMBERSHIP/PAGES/SCIENTIFIC%20WORKING%20GROUPS/CICR-.... Accessed March 16, 2017.
-
- Ashworth A. A synthetic lethal therapeutic approach: poly(ADP) ribose polymerase inhibitors for the treatment of cancers deficient in DNA double-strand break repair. J. Clin. Oncol. 2008;26:3785–3790. - PubMed
-
- Baell J.B., Holloway G.A. New substructure filters for removal of pan assay interference compounds (PAINS) from screening libraries and for their exclusion in bioassays. J. Med. Chem. 2010;53:2719–2740. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

