Analytical methodologies for broad metabolite coverage of exhaled breath condensate
- PMID: 28697414
- PMCID: PMC5573623
- DOI: 10.1016/j.jchromb.2017.06.038
Analytical methodologies for broad metabolite coverage of exhaled breath condensate
Abstract
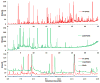
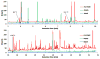
Breath analysis has been gaining popularity as a non-invasive technique that is amenable to a broad range of medical uses. One of the persistent problems hampering the wide application of the breath analysis method is measurement variability of metabolite abundances stemming from differences in both sampling and analysis methodologies used in various studies. Mass spectrometry has been a method of choice for comprehensive metabolomic analysis. For the first time in the present study, we juxtapose the most commonly employed mass spectrometry-based analysis methodologies and directly compare the resultant coverages of detected compounds in exhaled breath condensate in order to guide methodology choices for exhaled breath condensate analysis studies. Four methods were explored to broaden the range of measured compounds across both the volatile and non-volatile domain. Liquid phase sampling with polyacrylate Solid-Phase MicroExtraction fiber, liquid phase extraction with a polydimethylsiloxane patch, and headspace sampling using Carboxen/Polydimethylsiloxane Solid-Phase MicroExtraction (SPME) followed by gas chromatography mass spectrometry were tested for the analysis of volatile fraction. Hydrophilic interaction liquid chromatography and reversed-phase chromatography high performance liquid chromatography mass spectrometry were used for analysis of non-volatile fraction. We found that liquid phase breath condensate extraction was notably superior compared to headspace extraction and differences in employed sorbents manifested altered metabolite coverages. The most pronounced effect was substantially enhanced metabolite capture for larger, higher-boiling compounds using polyacrylate SPME liquid phase sampling. The analysis of the non-volatile fraction of breath condensate by hydrophilic and reverse phase high performance liquid chromatography mass spectrometry indicated orthogonal metabolite coverage by these chromatography modes. We found that the metabolite coverage could be enhanced significantly with the use of organic solvent as a device rinse after breath sampling to collect the non-aqueous fraction as opposed to neat breath condensate sample. Here, we show the detected ranges of compounds in each case and provide a practical guide for methodology selection for optimal detection of specific compounds.
Keywords: Exhaled breath condensate (EBC); Gas chromatography mass spectrometry (GC/MS); High performance liquid chromatography mass spectrometry (HPLC/MS); Hydrophilic interaction liquid chromatography (HILIC); Metabolites; Reversed-phase liquid chromatography (RP).
Copyright © 2017 Elsevier B.V. All rights reserved.
Figures



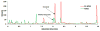
References
-
- Amann A, Smith D. Volatile Biomarkers Non-Invasive Diagnosis in Physiology and Medicine. Elsevier; China: 2013. pp. 129–154.
-
- Modak AS. Breath biomarkers for personalized medicine. Pers Med. 2010;7(6):643–653. - PubMed
-
- Phillips M. How to analyze breath and make sense of the data: a personal view. World Scientific Publishing Co. Pte. Ltd; 2005.
-
- Risby TH, Solga SF. Current status of clinical breath analysis. Appl Phys B Lasers and Optics. 2006;85(2):421–426.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

