Differential virulence and tsetse fly transmissibility of Trypanosoma congolense and Trypanosoma brucei strains
- PMID: 28697609
- PMCID: PMC6238703
- DOI: 10.4102/ojvr.v84i1.1412
Differential virulence and tsetse fly transmissibility of Trypanosoma congolense and Trypanosoma brucei strains
Abstract
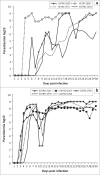
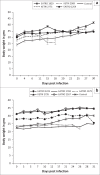
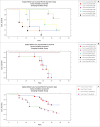
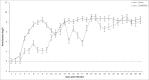
African animal trypanosomiasis causes significant economic losses in sub-Saharan African countries because of livestock mortalities and reduced productivity. Trypanosomes, the causative agents, are transmitted by tsetse flies (Glossina spp.). In the current study, we compared and contrasted the virulence characteristics of five Trypanosoma congolense and Trypanosoma brucei isolates using groups of Swiss white mice (n = 6). We further determined the vectorial capacity of Glossina pallidipes, for each of the trypanosome isolates. Results showed that the overall pre-patent (PP) periods were 8.4 ± 0.9 (range, 4-11) and 4.5 ± 0.2 (range, 4-6) for T. congolense and T. brucei isolates, respectively (p < 0.01). Despite the longer mean PP, T. congolense-infected mice exhibited a significantly (p < 0.05) shorter survival time than T. brucei-infected mice, indicating greater virulence. Differences were also noted among the individual isolates with T. congolense KETRI 2909 causing the most acute infection of the entire group with a mean ± standard error survival time of 9 ± 2.1 days. Survival time of infected tsetse flies and the proportion with mature infections at 30 days post-exposure to the infective blood meals varied among isolates, with subacute infection-causing T. congolense EATRO 1829 and chronic infection-causing T. brucei EATRO 2267 isolates showing the highest mature infection rates of 38.5% and 23.1%, respectively. Therefore, our study provides further evidence of occurrence of differences in virulence and transmissibility of eastern African trypanosome strains and has identified two, T. congolense EATRO 1829 and T. brucei EATRO 2267, as suitable for tsetse infectivity and transmissibility experiments.
Conflict of interest statement
The authors declare that they have no financial or personal relationships that may have inappropriately influenced them in writing this article.
Figures

References
-
- Bengaly Z., Sidibe I., Boly H., Sawadogo L. & Desquesnes M, 2002, ‘Comparative pathogenicity of three genetically distinct Trypanosoma congolense-types in inbred Balb/c mice’, Veterinary Parasitology 105, 111–118. https://doi.org/10.1016/S0304-4017(01)00609-4 - DOI - PubMed
-
- Biryomumaisho S. & Katunguka-Rwakishaya E, 2007, ‘The pathogenesis of anaemia in goats experimentally infected with Trypanosoma congolense or Trypanosoma brucei: Use of the myeloid:erythroid ratio’, Veterinary Parasitology 143, 354357 https://doi.org/10.1016/j.vetpar.2006.08.030 - DOI - PubMed
-
- Brun R., Hecker H. & Lun Z, 1998, ‘Trypanosoma evansi and T. equiperdum: Distribution, biology, treatment and phylogenetic relationship (a review)’, Veterinary Parasitology 79, 95–107. https://doi.org/10.1016/S0304-4017(98)00146-0 - DOI - PubMed
-
- Ciosi M., Masiga D. & Cmr T, 2014, ‘Laboratory colonisation and genetic bottlenecks in the tsetse fly glossina pallidipes’, PLOS Neglected Tropical Diseases 8, e2697 https://doi.org/10.1371/journal.pntd.0002697 - DOI - PMC - PubMed
-
- David B.J. & McCulloch R, 2001, ‘Antigenic variation in trypanosomes: Enhanced phenotypic variation in a eukaryotic parasite’, Advances in Parasitology 49, 2–70. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
