The impact of the time of drug administration on the effectiveness of combined treatment of hypercholesterolemia with Rosuvastatin and Ezetimibe (RosEze): study protocol for a randomized controlled trial
- PMID: 28697767
- PMCID: PMC5504756
- DOI: 10.1186/s13063-017-2047-8
The impact of the time of drug administration on the effectiveness of combined treatment of hypercholesterolemia with Rosuvastatin and Ezetimibe (RosEze): study protocol for a randomized controlled trial
Abstract
Background: Hypercholesterolemia is one of the main risk factors for cardiovascular disease. The first line treatment for hypercholesterolemia is statin therapy. When the expected low-density lipoprotein cholesterol (LDL-C) concentration is not achieved, the pharmacotherapy may be extended by combining the statin with the cholesterol absorption inhibitor ezetimibe.
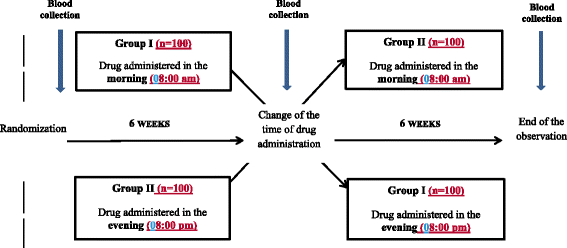
Methods/design: The study is designed as a randomized, open-label, single-center, crossover study evaluating the effectiveness of combined therapy with rosuvastatin and ezetimibe for hypercholesterolemia. The study is planned to include 200 patients with hypercholesterolemia ineffectively treated with statins for at least 6 weeks. After enrollment participants are randomized into one of two arms receiving rosuvastatin and ezetimibe. In the first arm the study drug is administered in the morning (8:00 am) for 6 weeks and then in the evening for the next 6 weeks; in the second arm the study drug is administered at first in the evening (8:00 pm) for the first 6 weeks and then in the morning for the following 6 weeks. In order to minimize non-adherence to the treatment, all patients will receive the study drug free of charge. The primary outcome of the study is change in LDL-C at 6 and 12 weeks of the treatment, depending on the time of day of study drug administration. The secondary endpoints include change in total cholesterol, high-density lipoprotein (HDL) cholesterol, triglycerides, apolipoproteins ApoB and Apo AI, non-HDL cholesterol, small, dense (sd)-LDL cholesterol, lipoprotein(a), glucose, glycated hemoglobin, high-sensitivity C-reactive protein, aspartate aminotransferase, alanine aminotransferase, gamma-glutamyl transferase, and creatine kinase at 6 and 12 weeks of the study drug treatment, as well as assessment of plasma fluorescence using stationary and time-resolved fluorescence spectroscopy at baseline and at 6 and 12 weeks of the therapy.
Discussion: The RosEze trial is expected to demonstrate whether there is a significant difference in the effectiveness of the lipid-lowering therapy in reducing the concentration of cholesterol when the medications are taken in the morning compared with the evening time of day.
Trial registration: ClinicalTrials.gov, NCT02772640 . Registered on 28 March 2016.
Keywords: Adherence; Cardiovascular risk; Coronary artery disease; Dyslipidemia; Ezetimibe; Hydroxymethylglutaryl-CoA; Hypercholesterolemia; LDL-C; Morning/evening dosing; Rosuvastatin.
Conflict of interest statement
Ethics approval and consent to participate
The protocol of the study was approved by the Ethics Committee of Nicolaus Copernicus University in Toruń, Ludwik Rydygier Collegium Medicum in Bydgoszcz (approval number KB 618/2015). Each participant needs to sign a written informed consent before enrollment into the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Catapano AL, Graham I, De Backer G, Wiklund O, Chapman MJ, Drexel H, et al. 2016 ESC/EAS Guidelines for the Management of Dyslipidaemias: The Task Force for the Management of Dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS) Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR) Atherosclerosis. 2016;253:281–344. doi: 10.1016/j.atherosclerosis.2016.08.018. - DOI - PubMed
-
- Eckel RH, Jakicic JM, Ard JD, de Jesus JM, Houston Miller N, Hubbard VS, et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 Suppl 2):S76–99. doi: 10.1161/01.cir.0000437740.48606.d1. - DOI - PubMed
-
- Dattilo AM, Kris-Etherton PM. Effects of weight reduction on blood lipids and lipoproteins: a meta-analysis. Am J Clin Nutr. 1992;56:320–8. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous