Control of lupus nephritis by changes of gut microbiota
- PMID: 28697806
- PMCID: PMC5505136
- DOI: 10.1186/s40168-017-0300-8
Control of lupus nephritis by changes of gut microbiota
Abstract
Background: Systemic lupus erythematosus, characterized by persistent inflammation, is a complex autoimmune disorder with no known cure. Immunosuppressants used in treatment put patients at a higher risk of infections. New knowledge of disease modulators, such as symbiotic bacteria, can enable fine-tuning of parts of the immune system, rather than suppressing it altogether.
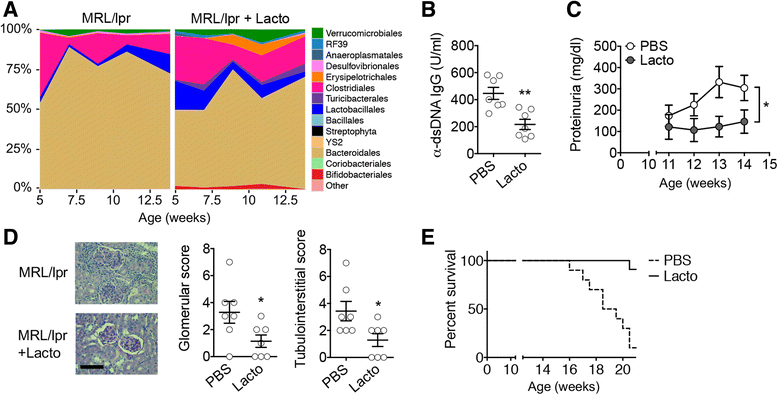
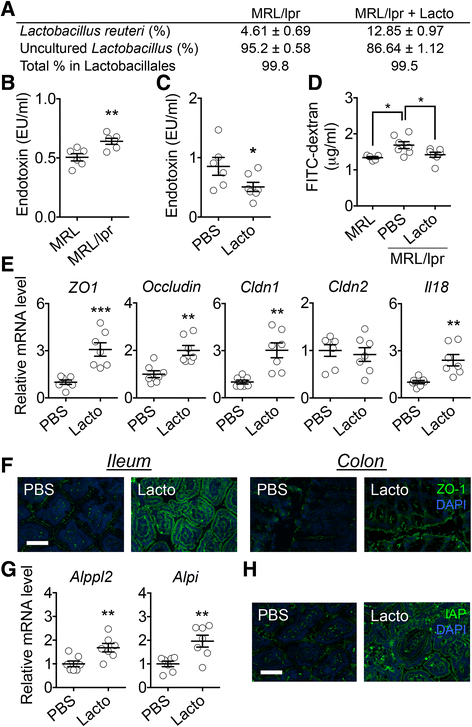
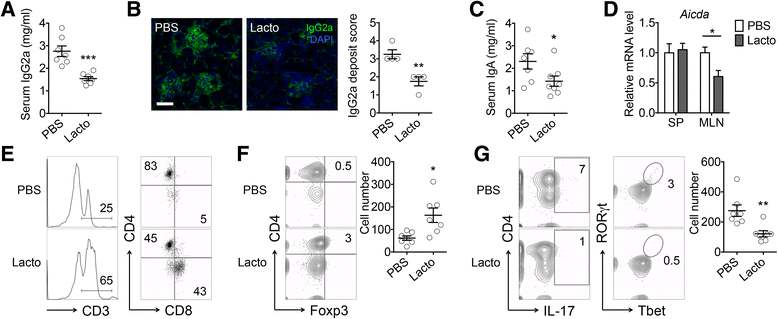
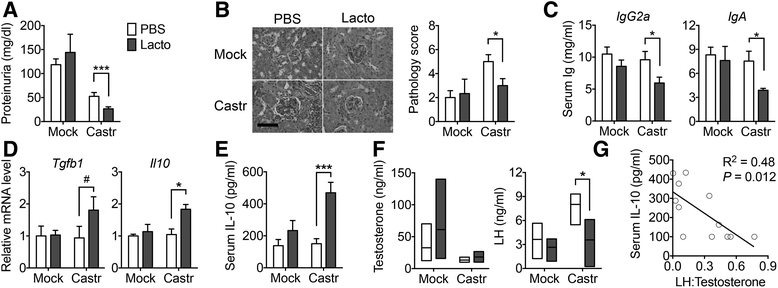
Results: Dysbiosis of gut microbiota promotes autoimmune disorders that damage extraintestinal organs. Here we report a role of gut microbiota in the pathogenesis of renal dysfunction in lupus. Using a classical model of lupus nephritis, MRL/lpr, we found a marked depletion of Lactobacillales in the gut microbiota. Increasing Lactobacillales in the gut improved renal function of these mice and prolonged their survival. We used a mixture of 5 Lactobacillus strains (Lactobacillus oris, Lactobacillus rhamnosus, Lactobacillus reuteri, Lactobacillus johnsonii, and Lactobacillus gasseri), but L. reuteri and an uncultured Lactobacillus sp. accounted for most of the observed effects. Further studies revealed that MRL/lpr mice possessed a "leaky" gut, which was reversed by increased Lactobacillus colonization. Lactobacillus treatment contributed to an anti-inflammatory environment by decreasing IL-6 and increasing IL-10 production in the gut. In the circulation, Lactobacillus treatment increased IL-10 and decreased IgG2a that is considered to be a major immune deposit in the kidney of MRL/lpr mice. Inside the kidney, Lactobacillus treatment also skewed the Treg-Th17 balance towards a Treg phenotype. These beneficial effects were present in female and castrated male mice, but not in intact males, suggesting that the gut microbiota controls lupus nephritis in a sex hormone-dependent manner.
Conclusions: This work demonstrates essential mechanisms on how changes of the gut microbiota regulate lupus-associated immune responses in mice. Future studies are warranted to determine if these results can be replicated in human subjects.
Keywords: Autoimmunity; Gut microbiota; Leaky gut; Lupus.
Conflict of interest statement
Ethics approval
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Institutional Animal Care and Use Committee (IACUC) of Virginia Tech College of Veterinary Medicine (Animal Welfare Assurance Number: A3208-01). For anesthesia and euthanasia, isoflurane and CO2 were used, respectively, according to the IACUC protocol.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

