Imaging at ultrahigh magnetic fields: History, challenges, and solutions
- PMID: 28698108
- PMCID: PMC5758441
- DOI: 10.1016/j.neuroimage.2017.07.007
Imaging at ultrahigh magnetic fields: History, challenges, and solutions
Abstract
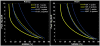
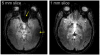
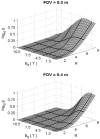
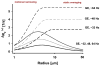
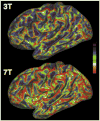
Following early efforts in applying nuclear magnetic resonance (NMR) spectroscopy to study biological processes in intact systems, and particularly since the introduction of 4 T human scanners circa 1990, rapid progress was made in imaging and spectroscopy studies of humans at 4 T and animal models at 9.4 T, leading to the introduction of 7 T and higher magnetic fields for human investigation at about the turn of the century. Work conducted on these platforms has provided numerous technological solutions to challenges posed at these ultrahigh fields, and demonstrated the existence of significant advantages in signal-to-noise ratio and biological information content. Primary difference from lower fields is the deviation from the near field regime at the radiofrequencies (RF) corresponding to hydrogen resonance conditions. At such ultrahigh fields, the RF is characterized by attenuated traveling waves in the human body, which leads to image non-uniformities for a given sample-coil configuration because of destructive and constructive interferences. These non-uniformities were initially considered detrimental to progress of imaging at high field strengths. However, they are advantageous for parallel imaging in signal reception and transmission, two critical technologies that account, to a large extend, for the success of ultrahigh fields. With these technologies and improvements in instrumentation and imaging methods, today ultrahigh fields have provided unprecedented gains in imaging of brain function and anatomy, and started to make inroads into investigation of the human torso and extremities. As extensive as they are, these gains still constitute a prelude to what is to come given the increasingly larger effort committed to ultrahigh field research and development of ever better instrumentation and techniques.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures
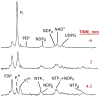
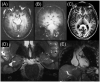
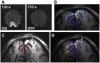

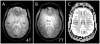




References
-
- Abduljalil A, Kangarlu A, Robitaille PML. Experimental Nuclear Magnetic Resonance Conference (ENC) Orlando, FL: 1999. Ultra-High field magnetic resonance imaging; p. 110.
-
- Ackerman JJ, Bore PJ, Gadian DG, Grove TH, Radda GK. N.m.r studies of metabolism in perfused organs. Philos Trans R Soc Lond B Biol Sci. 1980a;289:425–436. - PubMed
-
- Ackerman JJ, Grove TH, Wong GG, Gadian DG, Radda GK. Mapping of metabolites in whole animals by 31P NMR using surface coils. Nature. 1980b;283:167–170. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

