Effectiveness of Digital Medicines to Improve Clinical Outcomes in Patients with Uncontrolled Hypertension and Type 2 Diabetes: Prospective, Open-Label, Cluster-Randomized Pilot Clinical Trial
- PMID: 28698169
- PMCID: PMC5527253
- DOI: 10.2196/jmir.7833
Effectiveness of Digital Medicines to Improve Clinical Outcomes in Patients with Uncontrolled Hypertension and Type 2 Diabetes: Prospective, Open-Label, Cluster-Randomized Pilot Clinical Trial
Abstract
Background: Hypertension and type 2 diabetes mellitus are major modifiable risk factors for cardiac, cerebrovascular, and kidney diseases. Reasons for poor disease control include nonadherence, lack of patient engagement, and therapeutic inertia.
Objective: The aim of this study was to assess the impact on clinic-measured blood pressure (BP) and glycated hemoglobin (HbA1c) using a digital medicine offering (DMO) that measures medication ingestion adherence, physical activity, and rest using digital medicines (medication taken with ingestible sensor), wearable sensor patches, and a mobile device app.
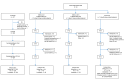
Methods: Participants with elevated systolic BP (SBP ≥140 mm Hg) and HbA1c (≥7%) failing antihypertensive (≥2 medications) and oral diabetes therapy were enrolled in this three-arm, 12-week, cluster-randomized study. Participants used DMO (includes digital medicines, the wearable sensor patch, and the mobile device app) for 4 or 12 weeks or received usual care based on site randomization. Providers in the DMO arms could review the DMO data via a Web portal. In all three arms, providers were instructed to make medical decisions (medication titration, adherence counseling, education, and lifestyle coaching) on all available clinical information at each visit. Primary outcome was change in SBP at week 4. Other outcomes included change in SBP and HbA1c at week 12, and low-density lipoprotein cholesterol (LDL-C) and diastolic blood pressure (DBP) at weeks 4 and 12, as well as proportion of patients at BP goal (<140/90 mm Hg) at weeks 4 and 12, medical decisions, and medication adherence patterns.
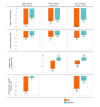
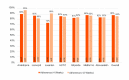
Results: Final analysis included 109 participants (12 sites; age: mean 58.7, SD years; female: 49.5%, 54/109; Hispanic: 45.9%, 50/109; income ≤ US $20,000: 56.9%, 62/109; and ≤ high school education: 52.3%, 57/109). The DMO groups had 80 participants (7 sites) and usual care had 29 participants (5 sites). At week 4, DMO resulted in a statistically greater SBP reduction than usual care (mean -21.8, SE 1.5 mm Hg vs mean -12.7, SE 2.8 mmHg; mean difference -9.1, 95% CI -14.0 to -3.3 mm Hg) and maintained a greater reduction at week 12. The DMO groups had greater reductions in HbA1c, DBP, and LDL-C, and a greater proportion of participants at BP goal at weeks 4 and 12 compared with usual care. The DMO groups also received more therapeutic interventions than usual care. Medication adherence was ≥80% while using the DMO. The most common adverse event was a self-limited rash at the wearable sensor site (12%, 10/82).
Conclusions: For patients failing hypertension and diabetes oral therapy, this DMO, which provides dose-by-dose feedback on medication ingestion adherence, can help lower BP, HbA1c, and LDL-C, and promote patient engagement and provider decision making.
Trial registration: Clinicaltrials.gov NCT02827630; https://clinicaltrials.gov/show/NCT02827630 (Archived by WebCite at http://www.webcitation.org/6rL8dW2VF).
Keywords: digital medicine; hypertension; patient engagement, medication adherence; therapeutic inertia; type 2 diabetes.
©Juan Frias, Naunihal Virdi, Praveen Raja, Yoona Kim, George Savage, Lars Osterberg. Originally published in the Journal of Medical Internet Research (http://www.jmir.org), 11.07.2017.
Conflict of interest statement
Conflicts of Interest: The sponsor, Proteus Digital Health, had a role in the design and conduct of the study; analysis and interpretation of the data; preparation, review, and approval of the manuscript; and decision to submit the manuscript for publication. The sponsor had no role in the collection or management of the study. JF reports consulting relationship with Proteus Digital Health, Johnson & Johnson, AstraZeneca, CeQur, and Sanofi. He also reports receiving study grants from Abbvie, Amgen, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, Johnson & Johnson, Merck, Novo Nordisk, Pfizer, and Sanofi. NV, PR, YK, and GS are employed by Proteus Digital Health. GS is also a cofounder, officer, and stock owner of Proteus Digital Health. LO reports a consulting relationship with Proteus Digital Health; his contribution to this publication was as a paid consultant and was not part of his Stanford University duties or responsibilities.
Figures

References
-
- Centers for Disease Control and Prevention (CDC) Vital signs: prevalence, treatment, and control of hypertension--United States, 1999-2002 and 2005-2008. MMWR Morb Mortal Wkly Rep. 2011 Feb 04;60(4):103–108. https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6004a4.htm - PubMed
-
- Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P, American Heart Association Statistics Committee Stroke Statistics Subcommittee Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation. 2017 Mar 07;135(10):e146–e603. doi: 10.1161/CIR.0000000000000485. - DOI - PMC - PubMed
-
- Heidenreich PA, Trogdon JG, Khavjou OA, Butler J, Dracup K, Ezekowitz MD, Finkelstein EA, Hong Y, Johnston SC, Khera A, Lloyd-Jones DM, Nelson SA, Nichol G, Orenstein D, Wilson PW, Woo YJ, American Heart Association Advocacy Coordinating Committee. Stroke C, Council on Cardiovascular Radiology and Intervention. Council on Clinical Cardiology. Council OE, Council OA, Thrombosis and Vascular Biology. Council OC, Critical C, Perioperative and Resuscitation. Council on Cardiovascular Nursing. Council on the Kidney in Cardiovascular Disease. Council on Cardiovascular Surgery and Anesthesia. Interdisciplinary Council on Quality of Care and Outcomes Research Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011 Mar 1;123(8):933–944. doi: 10.1161/CIR.0b013e31820a55f5. http://circ.ahajournals.org/cgi/pmidlookup?view=long&pmid=21262990 - DOI - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

