Natural Compounds for the Treatment of Psoriatic Arthritis: A Proposal Based on Multi-Targeted Osteoclastic Regulation and on a Preclinical Study
- PMID: 28698171
- PMCID: PMC5527251
- DOI: 10.2196/resprot.7636
Natural Compounds for the Treatment of Psoriatic Arthritis: A Proposal Based on Multi-Targeted Osteoclastic Regulation and on a Preclinical Study
Abstract
Background: Psoriatic arthritis (PsA) is a chronic inflammatory arthritis affecting approximately 2% to 3% of the population globally, and is characterized by both peripheral articular manifestations and axial skeletal involvement. Conventional therapies for PsA have not been fully satisfactory, though natural products (NPs) have been shown to be highly effective and represent important treatment options for psoriasis. PsA is a multigenic autoimmune disease with both environmental and genetic factors contributing to its pathogenesis. Accordingly, it is likely that the use of natural compounds with a multi-targeted approach will enable us to develop better therapies for PsA and related disorders.
Objective: PsA, either on joint damage or on bone erosion, has been shown to respond to anti-psoriatic pharmacotherapy (APP), APP-like NPs, and their natural compounds. This study aims to uncover specific natural compounds for improved PsA remedies. Specifically, by targeting bone erosion caused by increased osteoclastic bone resorption, we aim to predict the key signaling pathways affected by natural compounds. Further, the study will explore their anti-arthritis effects using an in silico, in vitro, and in vivo approach. Following the signaling pathway prediction, a preclinical efficacy study on animal models will be undertaken. Collectively, this work will discover lead compounds with improved therapeutic effects on PsA.
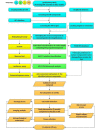
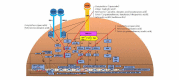
Methods: We hypothesize that 9 potential APP-like NPs will have therapeutic effects on arthritis via the modulation of osteoclast bone resorption and signaling pathways. For in silico identification, the Latin name of each NP will be identified using the Encyclopedia of Traditional Chinese Medicine (Encyclopedia of TCM). The biological targets of NPs will be predicted or screened using the Herbal Ingredients' Targets (HIT) database. With the designed search terms, DrugBank will be used to further filter the above biological targets. Protein ANnotation THrough Evolutionary Relationship (PANTHER) will be used to predict the pathways of the natural compound sources. Subsequently, an in vitro sample preparation including extraction, fractionation, isolation, purification, and bioassays with high-speed counter-current chromatography-high-performance liquid chromatography-diode array detection (HSCCC-HPLC-DAD) will be carried out for each identified natural source. In vitro investigations into the effect of NPs on osteoclast signaling pathways will be performed. The experimental methods include cell viability assays, osteoclastogenesis and resorption pit assays, quantitative reverse transcription polymerase chain reaction (RT-PCR), western blot, and luciferase reporter gene assays. Finally, an in vivo preclinical efficacy on a collagen-induced arthritis rat model will be carried out using a treatment group (n=10), a control group (n=10), and a non-arthritis group (n=10). Main outcome measure assessments during intervention include daily macroscopic scores and a digital calipers measurement. Post-treatment tissue measurements will be analyzed by serological testing, radiographic imaging, and histopathological assessment.
Results: Studies are currently underway to evaluate the in silico data and the in vitro effects of compounds on osteoclastogenesis and bone resorption. The preclinical study is expected to start a year following completion of the in silico analysis.
Conclusions: The in silico rapid approach is proposed as a more general method for adding value to the results of a systematic review of NPs. More importantly, the proposed study builds on a multi-targeted approach for the identification of natural compounds for future drug discovery. This innovative approach is likely to be more precise, efficient, and compatible to identify the novel natural compounds for effective treatment of PsA.
Keywords: RANKL signaling pathways; anti-psoriatic pharmacotherapy (APP); arthritis; in silico screening; multi-target; natural compounds; osteoclast; preclinical study.
©Shiqiang Deng, Jianwen Cheng, Jinmin Zhao, Felix Yao, Jiake Xu. Originally published in JMIR Research Protocols (http://www.researchprotocols.org), 11.07.2017.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures

Similar articles
-
Lymphocytes and synovial fluid fibroblasts support osteoclastogenesis through RANKL, TNFalpha, and IL-7 in an in vitro model derived from human psoriatic arthritis.J Pathol. 2007 May;212(1):47-55. doi: 10.1002/path.2153. J Pathol. 2007. PMID: 17370327
-
More advantages in detecting bone and soft tissue metastases from prostate cancer using 18F-PSMA PET/CT.Hell J Nucl Med. 2019 Jan-Apr;22(1):6-9. doi: 10.1967/s002449910952. Epub 2019 Mar 7. Hell J Nucl Med. 2019. PMID: 30843003
-
Safety and nutritional assessment of GM plants and derived food and feed: the role of animal feeding trials.Food Chem Toxicol. 2008 Mar;46 Suppl 1:S2-70. doi: 10.1016/j.fct.2008.02.008. Epub 2008 Feb 13. Food Chem Toxicol. 2008. PMID: 18328408 Review.
-
Imaging in psoriatic arthritis: established methods and emerging techniques.Ther Adv Musculoskelet Dis. 2024 Oct 16;16:1759720X241288060. doi: 10.1177/1759720X241288060. eCollection 2024. Ther Adv Musculoskelet Dis. 2024. PMID: 39421802 Free PMC article. Review.
-
[Mechanism prediction of Simiao Yongan Decoction in treatment of psoriasis arthritis based on network pharmacology].Zhongguo Zhong Yao Za Zhi. 2020 Jun;45(11):2611-2618. doi: 10.19540/j.cnki.cjcmm.20200225.401. Zhongguo Zhong Yao Za Zhi. 2020. PMID: 32627496 Chinese.
Cited by
-
Astilbin prevents bone loss in ovariectomized mice through the inhibition of RANKL-induced osteoclastogenesis.J Cell Mol Med. 2019 Dec;23(12):8355-8368. doi: 10.1111/jcmm.14713. Epub 2019 Oct 11. J Cell Mol Med. 2019. PMID: 31603626 Free PMC article.
-
Patchouli Alcohol Modulates the Pregnancy X Receptor/Toll-like Receptor 4/Nuclear Factor Kappa B Axis to Suppress Osteoclastogenesis.Front Pharmacol. 2021 Jun 8;12:684976. doi: 10.3389/fphar.2021.684976. eCollection 2021. Front Pharmacol. 2021. PMID: 34177594 Free PMC article.
-
Madecassoside inhibits estrogen deficiency-induced osteoporosis by suppressing RANKL-induced osteoclastogenesis.J Cell Mol Med. 2019 Jan;23(1):380-394. doi: 10.1111/jcmm.13942. Epub 2018 Oct 19. J Cell Mol Med. 2019. PMID: 30338925 Free PMC article.
-
Natural Compound Mixture, Containing Emodin, Genipin, Chlorogenic Acid, Cimigenoside, and Ginsenoside Rb1, Ameliorates Psoriasis-Like Skin Lesions by Suppressing Inflammation and Proliferation in Keratinocytes.Evid Based Complement Alternat Med. 2020 Oct 22;2020:9416962. doi: 10.1155/2020/9416962. eCollection 2020. Evid Based Complement Alternat Med. 2020. PMID: 33149756 Free PMC article.
References
-
- Xue Y, Jiang L, Cheng Q, Chen H, Yu Y, Lin Y, Yang X, Kong N, Zhu X, Xu X, Wan W, Zou H. Adipokines in psoriatic arthritis patients: the correlations with osteoclast precursors and bone erosions. PLoS One. 2012;7(10):e46740. doi: 10.1371/journal.pone.0046740. http://dx.plos.org/10.1371/journal.pone.0046740 - DOI - DOI - PMC - PubMed
-
- Chen X, Decker M. Multi-target compounds acting in the central nervous system designed from natural products. Curr Med Chem. 2013;20(13):1673–85. - PubMed
-
- Kwak HB, Lee BK, Oh J, Yeon J, Choi S, Cho HJ, Lee MS, Kim J, Bae J, Kim SH, Kim HS. Inhibition of osteoclast differentiation and bone resorption by rotenone, through down-regulation of RANKL-induced c-Fos and NFATc1 expression. Bone. 2010 Mar;46(3):724–31. doi: 10.1016/j.bone.2009.10.042. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

