Interferon Regulatory Factor 5 Controls Necrotic Core Formation in Atherosclerotic Lesions by Impairing Efferocytosis
- PMID: 28698173
- PMCID: PMC5598917
- DOI: 10.1161/CIRCULATIONAHA.117.027844
Interferon Regulatory Factor 5 Controls Necrotic Core Formation in Atherosclerotic Lesions by Impairing Efferocytosis
Abstract
Background: Myeloid cells are central to atherosclerotic lesion development and vulnerable plaque formation. Impaired ability of arterial phagocytes to uptake apoptotic cells (efferocytosis) promotes lesion growth and establishment of a necrotic core. The transcription factor interferon regulatory factor (IRF)-5 is an important modulator of myeloid function and programming. We sought to investigate whether IRF5 affects the formation and phenotype of atherosclerotic lesions.
Methods: We investigated the role of IRF5 in atherosclerosis in 2 complementary models. First, atherosclerotic lesion development in hyperlipidemic apolipoprotein E-deficient (ApoE-/-) mice and ApoE-/- mice with a genetic deletion of IRF5 (ApoE-/-Irf5-/-) was compared and then lesion development was assessed in a model of shear stress-modulated vulnerable plaque formation.
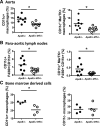
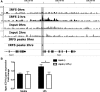
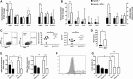
Results: Both lesion and necrotic core size were significantly reduced in ApoE-/-Irf5-/- mice compared with IRF5-competent ApoE-/- mice. Necrotic core size was also reduced in the model of shear stress-modulated vulnerable plaque formation. A significant loss of CD11c+ macrophages was evident in ApoE-/-Irf5-/- mice in the aorta, draining lymph nodes, and bone marrow cell cultures, indicating that IRF5 maintains CD11c+ macrophages in atherosclerosis. Moreover, we revealed that the CD11c gene is a direct target of IRF5 in macrophages. In the absence of IRF5, CD11c- macrophages displayed a significant increase in expression of the efferocytosis-regulating integrin-β3 and its ligand milk fat globule-epidermal growth factor 8 protein and enhanced efferocytosis in vitro and in situ.
Conclusions: IRF5 is detrimental in atherosclerosis by promoting the maintenance of proinflammatory CD11c+ macrophages within lesions and controlling the expansion of the necrotic core by impairing efferocytosis.
Keywords: CD11c; IRF5; atherosclerosis; efferocytosis; macrophages.
© 2017 The Authors.
Figures





References
-
- Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Barker-Collo S, Bartels DH, Bell ML, Benjamin EJ, Bennett D, Bhalla K, Bikbov B, Bin Abdulhak A, Birbeck G, Blyth F, Bolliger I, Boufous S, Bucello C, Burch M, Burney P, Carapetis J, Chen H, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahodwala N, De Leo D, Degenhardt L, Delossantos A, Denenberg J, Des Jarlais DC, Dharmaratne SD, Dorsey ER, Driscoll T, Duber H, Ebel B, Erwin PJ, Espindola P, Ezzati M, Feigin V, Flaxman AD, Forouzanfar MH, Fowkes FG, Franklin R, Fransen M, Freeman MK, Gabriel SE, Gakidou E, Gaspari F, Gillum RF, Gonzalez-Medina D, Halasa YA, Haring D, Harrison JE, Havmoeller R, Hay RJ, Hoen B, Hotez PJ, Hoy D, Jacobsen KH, James SL, Jasrasaria R, Jayaraman S, Johns N, Karthikeyan G, Kassebaum N, Keren A, Khoo JP, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Lipnick M, Lipshultz SE, Ohno SL, Mabweijano J, MacIntyre MF, Mallinger L, March L, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGrath J, Mensah GA, Merriman TR, Michaud C, Miller M, Miller TR, Mock C, Mocumbi AO, Mokdad AA, Moran A, Mulholland K, Nair MN, Naldi L, Narayan KM, Nasseri K, Norman P, O’Donnell M, Omer SB, Ortblad K, Osborne R, Ozgediz D, Pahari B, Pandian JD, Rivero AP, Padilla RP, Perez-Ruiz F, Perico N, Phillips D, Pierce K, Pope CA, 3rd, Porrini E, Pourmalek F, Raju M, Ranganathan D, Rehm JT, Rein DB, Remuzzi G, Rivara FP, Roberts T, De Leon FR, Rosenfeld LC, Rushton L, Sacco RL, Salomon JA, Sampson U, Sanman E, Schwebel DC, Segui-Gomez M, Shepard DS, Singh D, Singleton J, Sliwa K, Smith E, Steer A, Taylor JA, Thomas B, Tleyjeh IM, Towbin JA, Truelsen T, Undurraga EA, Venketasubramanian N, Vijayakumar L, Vos T, Wagner GR, Wang M, Wang W, Watt K, Weinstock MA, Weintraub R, Wilkinson JD, Woolf AD, Wulf S, Yeh PH, Yip P, Zabetian A, Zheng ZJ, Lopez AD, Murray CJ, AlMazroa MA, Memish ZA. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. - PMC - PubMed
-
- Gu L, Okada Y, Clinton SK, Gerard C, Sukhova GK, Libby P, Rollins BJ. Absence of monocyte chemoattractant protein-1 reduces atherosclerosis in low density lipoprotein receptor-deficient mice. Mol Cell. 1998;2:275–281. - PubMed
-
- Chinetti-Gbaguidi G, Colin S, Staels B. Macrophage subsets in atherosclerosis. Nat Rev Cardiol. 2015;12:10–17. doi: 10.1038/nrcardio.2014.173. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

