Entinostat Neutralizes Myeloid-Derived Suppressor Cells and Enhances the Antitumor Effect of PD-1 Inhibition in Murine Models of Lung and Renal Cell Carcinoma
- PMID: 28698201
- PMCID: PMC5723438
- DOI: 10.1158/1078-0432.CCR-17-0741
Entinostat Neutralizes Myeloid-Derived Suppressor Cells and Enhances the Antitumor Effect of PD-1 Inhibition in Murine Models of Lung and Renal Cell Carcinoma
Abstract
Purpose: Recent advances in immunotherapy highlight the antitumor effects of immune checkpoint inhibition despite a relatively limited subset of patients receiving clinical benefit. The selective class I histone deacetylase inhibitor entinostat has been reported to have immunomodulatory activity including targeting of immune suppressor cells in the tumor microenvironment. Thus, we decided to assess whether entinostat could enhance anti-PD-1 treatment and investigate those alterations in the immunosuppressive tumor microenvironment that contribute to the combined antitumor activity.
Experimental design: We utilized syngeneic mouse models of lung (LLC) and renal cell (RENCA) carcinoma and assessed immune correlates, tumor growth, and survival following treatment with entinostat (5 or 10 mg/kg, p.o.) and a PD-1 inhibitor (10 and 20 mg/kg, s.c.).
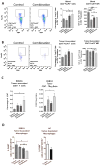
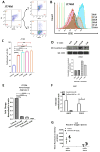
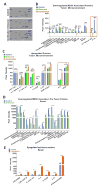
Results: Entinostat enhanced the antitumor effect of PD-1 inhibition in two syngeneic mouse tumor models by reducing tumor growth and increasing survival. Entinostat inhibited the immunosuppressive function of both polymorphonuclear (PMN)- and monocytic-myeloid derived suppressor cell (M-MDSC) populations. Analysis of MDSC response to entinostat revealed significantly reduced arginase-1, iNOS, and COX-2 levels, suggesting potential mechanisms for the altered function. We also observed significant alterations in cytokine/chemokine release in vivo with a shift toward a tumor-suppressive microenvironment.
Conclusions: Our results demonstrate that entinostat enhances the antitumor effect of PD-1 targeting through functional inhibition of MDSCs and a transition away from an immune-suppressive tumor microenvironment. These data provide a mechanistic rationale for the clinical testing and potential markers of response of this novel combination in solid tumor patients.
©2017 American Association for Cancer Research.
Conflict of interest statement
Figures



Similar articles
-
Class I histone deacetylase inhibitor entinostat suppresses regulatory T cells and enhances immunotherapies in renal and prostate cancer models.PLoS One. 2012;7(1):e30815. doi: 10.1371/journal.pone.0030815. Epub 2012 Jan 27. PLoS One. 2012. PMID: 22303460 Free PMC article.
-
Prim-O-glucosylcimifugin enhances the antitumour effect of PD-1 inhibition by targeting myeloid-derived suppressor cells.J Immunother Cancer. 2019 Aug 28;7(1):231. doi: 10.1186/s40425-019-0676-z. J Immunother Cancer. 2019. PMID: 31462297 Free PMC article.
-
Selective targeting of different populations of myeloid-derived suppressor cells by histone deacetylase inhibitors.Cancer Immunol Immunother. 2020 Sep;69(9):1929-1936. doi: 10.1007/s00262-020-02588-7. Epub 2020 May 20. Cancer Immunol Immunother. 2020. PMID: 32435850 Free PMC article.
-
Biomarkers for PD-1/PD-L1 Blockade Therapy in Non-Small-cell Lung Cancer: Is PD-L1 Expression a Good Marker for Patient Selection?Clin Lung Cancer. 2016 Sep;17(5):350-361. doi: 10.1016/j.cllc.2016.03.011. Epub 2016 Apr 6. Clin Lung Cancer. 2016. PMID: 27137346 Review.
-
Entinostat for the treatment of breast cancer.Expert Opin Investig Drugs. 2017 Aug;26(8):965-971. doi: 10.1080/13543784.2017.1353077. Epub 2017 Jul 24. Expert Opin Investig Drugs. 2017. PMID: 28718331 Review.
Cited by
-
Therapeutic Targets and Tumor Microenvironment in Colorectal Cancer.J Clin Med. 2021 May 25;10(11):2295. doi: 10.3390/jcm10112295. J Clin Med. 2021. PMID: 34070480 Free PMC article. Review.
-
The emerging role of epigenetic therapeutics in immuno-oncology.Nat Rev Clin Oncol. 2020 Feb;17(2):75-90. doi: 10.1038/s41571-019-0266-5. Epub 2019 Sep 23. Nat Rev Clin Oncol. 2020. PMID: 31548600 Free PMC article. Review.
-
Tuning the Tumor Myeloid Microenvironment to Fight Cancer.Front Immunol. 2019 Jul 25;10:1611. doi: 10.3389/fimmu.2019.01611. eCollection 2019. Front Immunol. 2019. PMID: 31402908 Free PMC article. Review.
-
Enhancing immune checkpoint blockade therapy of genitourinary malignancies by co-targeting PMN-MDSCs.Biochim Biophys Acta Rev Cancer. 2022 May;1877(3):188702. doi: 10.1016/j.bbcan.2022.188702. Epub 2022 Feb 25. Biochim Biophys Acta Rev Cancer. 2022. PMID: 35227829 Free PMC article.
-
Tumor-Infiltrating Immunosuppressive Cells in Cancer-Cell Plasticity, Tumor Progression and Therapy Response.Cancer Microenviron. 2019 Dec;12(2-3):119-132. doi: 10.1007/s12307-019-00232-2. Epub 2019 Oct 3. Cancer Microenviron. 2019. PMID: 31583529 Free PMC article.
References
-
- Shen L, Ciesielski M, Ramakrishnan S, Miles KM, Ellis L, Sotomayor P, et al. Class I histone deacetylase inhibitor entinostat suppresses regulatory T cells and enhances immunotherapies in renal and prostate cancer models. PloS one. 2012;7(1):e30815. doi: 10.1371/journal.pone.0030815. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

