Longitudinal characterisation of function and structure of Bietti crystalline dystrophy: report on a novel homozygous mutation in CYP4V2
- PMID: 28698241
- PMCID: PMC6208130
- DOI: 10.1136/bjophthalmol-2016-309696
Longitudinal characterisation of function and structure of Bietti crystalline dystrophy: report on a novel homozygous mutation in CYP4V2
Abstract
Background: Bietti crystalline dystrophy (BCD) is a rare inherited disorder characterised by fine crystalline deposits in the corneal limbus and retinal posterior pole. In 2004, mutations in the CYP4V2 gene were identified as the cause of BCD. Here, we describe the report of a homozygous point mutation in a patient with BCD and provide detailed characterisation of functional and structural changes over 20 years.
Methods: At regular intervals, the patient underwent repeat ophthalmic evaluations. DNA was extracted from buccal swabs, amplified by standard PCR and analysed for homology to the CYP4V2 sequence. Homology modelling was conducted using Iterative Threading ASSEmbly Refinement and molecular dynamics simulations using GROningen MAchine for Chemical Simulations.
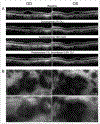
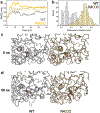
Results: The proband, a 47-year-old woman of German ancestry was diagnosed with crystalline retinopathy at age 25. Over the next 20 years, visual acuity and function gradually declined with progression of retinal pigment epithelium and choroidal atrophy. When first tested at 39 years of age, the multifocal electroretinogram (ERG) was markedly abnormal, more so for the right eye, whereas the full-field ERG was more symmetrical and lagged other measures of visual function. Gene sequencing showed a single C>T point mutation in exon 9 encoding a R400C amino acid change. Computational modelling suggests the mutation impairs function due to loss of a hydrogen bonding interaction with the propionate side chains of the haeme prosthetic group.
Conclusion: This is the first report of a homozygous R400C mutation in CYP4V2 with protein modelling showing high likelihood of enzyme dysfunction. The comprehensive long-term clinical follow-up provides insight into disease progression and highlights possible anti-inflammatory modulation of disease severity.
Keywords: Bietti crystalline dystrophy; CYP4V2; macular edema; protein modeling.
© Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2018. All rights reserved. No commercial use is permitted unless otherwise expressly granted.
Conflict of interest statement
Competing interests: Weleber is a consultant to Novartis, Pfizer and Wellstat; is a member of the scientific advisory board for Applied Genetic Technologies Corp; and serves on the scientific advisory board for the Foundation Fighting Blindness (the relationship has been reviewed and managed by Oregon Health and Science University). Dr Weleber also reports having received grants and personal fees from the Foundation Fighting Blindness and Applied Genetic Technologies Corp and other support from Sanofi, all outside the submitted work. In addition, Dr Weleber has a patent (US patent 8 657 446, method and apparatus for visual field monitoring, also known as Visual Field Monitoring and Analysis, or VFMA, which has not been issued).
Figures



References
-
- Ng DS, Lai TY, Ng TK, et al. Genetics of Bietti crystalline dystrophy. Asia Pac J Ophthalmol 2016;5:245–52. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
