Reversibility of Cardiac Function Predicts Outcome After Transcatheter Aortic Valve Replacement in Patients With Severe Aortic Stenosis
- PMID: 28698259
- PMCID: PMC5586298
- DOI: 10.1161/JAHA.117.005798
Reversibility of Cardiac Function Predicts Outcome After Transcatheter Aortic Valve Replacement in Patients With Severe Aortic Stenosis
Abstract
Background: Reversibility of left ventricular (LV) dysfunction in high-risk aortic stenosis patient and its impact on survival after transcatheter aortic valve replacement (TAVR) are unclear. We aimed to evaluate longitudinal changes of LV structure and function after TAVR and their impact on survival.
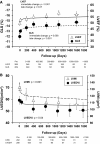
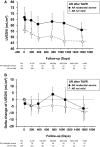
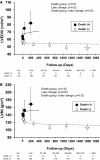
Methods and results: We studied 209 patients with aortic stenosis who underwent TAVR from May 2006 to December 2012. Echocardiograms were used to calculate LV end-diastolic volume index (LVEDVi), LV ejection fraction, LV mass index (LVMi), and global longitudinal strain before, immediately (<10 days), late (1-3 months), and yearly after TAVR. During a median follow-up of 1345 days, 118 patients died, with 26 dying within 1 year. Global longitudinal strain, LVEDVi, LV ejection fraction, and LVMi improved during follow-up. In patients who died during the first year, death was preceded by LVEDVi and LVMi increase. Multivariable longitudinal data analysis showed that aortic regurgitation at baseline, aortic regurgitation at 30 days, and initial LVEDVi were independent predictors of subsequent LVEDVi. In a joint analysis of longitudinal and survival data, baseline Society of Thoracic Surgeons score was predictive of survival, with no additive effect of longitudinal changes in LVEDVi, LVMi, global longitudinal strain, or LV ejection fraction. Presence of aortic regurgitation at 1 month after TAVR was the only predictor of 1-year survival.
Conclusions: LV reverse remodeling was observed after TAVR, whereas lack of LVEDVi and LVMi improvement was observed in patients who died during the first year after TAVR. Post-TAVR, aortic regurgitation blocks reverse remodeling and is associated with poor 1-year survival after TAVR.
Keywords: aortic valve stenosis; echocardiography; longitudinal strain; remodeling; transcatheter aortic valve implantation.
© 2017 The Authors. Published on behalf of the American Heart Association, Inc., by Wiley.
Figures

Similar articles
-
Transapical Transcatheter Aortic Valve Replacement Is Associated With Increased Cardiac Mortality in Patients With Left Ventricular Dysfunction: Insights From the PARTNER I Trial.JACC Cardiovasc Interv. 2017 Dec 11;10(23):2414-2422. doi: 10.1016/j.jcin.2017.09.023. JACC Cardiovasc Interv. 2017. PMID: 29217004 Clinical Trial.
-
Impact of Paravalvular Aortic Insufficiency on Left Ventricular Remodeling and Mortality after Transcatheter Aortic Valve Replacement.J Heart Valve Dis. 2016 May;25(3):301-308. J Heart Valve Dis. 2016. PMID: 27989040
-
Incremental Prognostic Use of Left Ventricular Global Longitudinal Strain in Asymptomatic/Minimally Symptomatic Patients With Severe Bioprosthetic Aortic Stenosis Undergoing Redo Aortic Valve Replacement.Circ Cardiovasc Imaging. 2017 Jun;10(6):e005942. doi: 10.1161/CIRCIMAGING.116.005942. Circ Cardiovasc Imaging. 2017. PMID: 28559420
-
Cardiac structural changes after transcatheter aortic valve replacement: systematic review and meta-analysis of cardiovascular magnetic resonance studies.J Cardiovasc Magn Reson. 2020 Jun 1;22(1):41. doi: 10.1186/s12968-020-00629-9. J Cardiovasc Magn Reson. 2020. PMID: 32475350 Free PMC article.
-
The Impact of Concomitant Mitral Regurgitation on Echocardiography Parameters After TransCatheter Aortic Valve Replacement: A Systematic Review and Meta-Analysis.Catheter Cardiovasc Interv. 2025 Jul;106(1):367-376. doi: 10.1002/ccd.31555. Epub 2025 Apr 24. Catheter Cardiovasc Interv. 2025. PMID: 40270122
Cited by
-
Left Ventricular Diastolic Dysfunction and Transcatheter Aortic Valve Replacement Outcomes: A Review.Cardiol Ther. 2019 Jun;8(1):21-28. doi: 10.1007/s40119-019-0134-5. Epub 2019 Mar 7. Cardiol Ther. 2019. PMID: 30847743 Free PMC article. Review.
-
Echocardiographic Evaluation after Transcatheter Aortic Valve Implantation: A Comprehensive Review.Life (Basel). 2023 Apr 24;13(5):1079. doi: 10.3390/life13051079. Life (Basel). 2023. PMID: 37240724 Free PMC article. Review.
-
Long-term prognostic value of the H2FPEF score in patients undergoing transcatheter aortic valve implantation.ESC Heart Fail. 2024 Aug;11(4):2159-2171. doi: 10.1002/ehf2.14773. Epub 2024 Apr 12. ESC Heart Fail. 2024. PMID: 38607328 Free PMC article.
-
Non-invasive myocardial work as an independent predictor of postprocedural NT-proBNP in elderly patients undergoing transcatheter aortic valve replacement.Geroscience. 2025 Jun;47(3):3311-3323. doi: 10.1007/s11357-024-01302-0. Epub 2024 Aug 8. Geroscience. 2025. PMID: 39115641 Free PMC article.
-
Left ventricular hypertrophy as a predictor of cardiovascular outcomes after transcatheter aortic valve replacement.ESC Heart Fail. 2023 Apr;10(2):1336-1346. doi: 10.1002/ehf2.14305. Epub 2023 Feb 1. ESC Heart Fail. 2023. PMID: 36725669 Free PMC article.
References
-
- Hill JA, Olson EN. Cardiac plasticity. N Engl J Med. 2008;358:1370–1380. - PubMed
-
- Heymans S, Schroen B, Vermeersch P, Milting H, Gao F, Kassner A, Gillijns H, Herijgers P, Flameng W, Carmeliet P, Van de Werf F, Pinto YM, Janssens S. Increased cardiac expression of tissue inhibitor of metalloproteinase‐1 and tissue inhibitor of metalloproteinase‐2 is related to cardiac fibrosis and dysfunction in the chronic pressure‐overloaded human heart. Circulation. 2005;112:1136–1144. - PubMed
-
- Shan K, Bick RJ, Poindexter BJ, Shimoni S, Letsou GV, Reardon MJ, Howell JF, Zoghbi WA, Nagueh SF. Relation of tissue Doppler derived myocardial velocities to myocardial structure and beta‐adrenergic receptor density in humans. J Am Coll Cardiol. 2000;36:891–896. - PubMed
-
- Leon MB, Smith CR, Mack MJ, Makkar RR, Svensson LG, Kodali SK, Thourani VH, Tuzcu EM, Miller DC, Herrmann HC, Doshi D, Cohen DJ, Pichard AD, Kapadia S, Dewey T, Babaliaros V, Szeto WY, Williams MR, Kereiakes D, Zajarias A, Greason KL, Whisenant BK, Hodson RW, Moses JW, Trento A, Brown DL, Fearon WF, Pibarot P, Hahn RT, Jaber WA, Anderson WN, Alu MC, Webb JG. Transcatheter or surgical aortic‐valve replacement in intermediate‐risk patients. N Engl J Med. 2016;374:1609–1620. - PubMed
-
- Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, Brown DL, Block PC, Guyton RA, Pichard AD, Bavaria JE, Herrmann HC, Douglas PS, Petersen JL, Akin JJ, Anderson WN, Wang D, Pocock S. Transcatheter aortic‐valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597–1607. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

