Zika Virus Persistently Infects and Is Basolaterally Released from Primary Human Brain Microvascular Endothelial Cells
- PMID: 28698279
- PMCID: PMC5513708
- DOI: 10.1128/mBio.00952-17
Zika Virus Persistently Infects and Is Basolaterally Released from Primary Human Brain Microvascular Endothelial Cells
Abstract
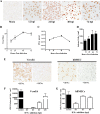
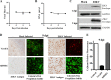
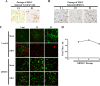
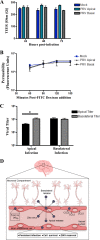
Zika virus (ZIKV) is a mosquito-borne Flavivirus that has emerged as the cause of encephalitis and fetal microencephaly in the Americas. ZIKV uniquely persists in human bodily fluids for up to 6 months, is sexually transmitted, and traverses the placenta and the blood-brain barrier (BBB) to damage neurons. Cells that support persistent ZIKV replication and mechanisms by which ZIKV establishes persistence remain enigmatic but central to ZIKV entry into protected neuronal compartments. The endothelial cell (EC) lining of capillaries normally constrains transplacental transmission and forms the BBB, which selectively restricts access of blood constituents to neurons. We found that ZIKV (strain PRVABC59) persistently infects and continuously replicates in primary human brain microvascular ECs (hBMECs), without cytopathology, for >9 days and following hBMEC passage. ZIKV did not permeabilize hBMECs but was released basolaterally from polarized hBMECs, suggesting a direct mechanism for ZIKV to cross the BBB. ZIKV-infected hBMECs were rapidly resistant to alpha interferon (IFN-α) and transiently induced, but failed to secrete, IFN-β and IFN-λ. Global transcriptome analysis determined that ZIKV constitutively induced IFN regulatory factor 7 (IRF7), IRF9, and IFN-stimulated genes (ISGs) 1 to 9 days postinfection, despite persistently replicating in hBMECs. ZIKV constitutively induced ISG15, HERC5, and USP18, which are linked to hepatitis C virus (HCV) persistence and IFN regulation, chemokine CCL5, which is associated with immunopathogenesis, as well as cell survival factors. Our results reveal that hBMECs act as a reservoir of persistent ZIKV replication, suggest routes for ZIKV to cross hBMECs into neuronal compartments, and define novel mechanisms of ZIKV persistence that can be targeted to restrict ZIKV spread.IMPORTANCE ZIKV persists in patients, crossing placental and neuronal barriers, damaging neurons, and causing fetal microencephaly. We found that ZIKV persistently infects brain endothelial cells that normally protect neurons from viral exposure. hBMECs are not damaged by ZIKV infection and, analogous to persistent HCV infection, ZIKV constitutively induces and evades antiviral ISG and IFN responses to continuously replicate in hBMECs. As a result, hBMECs provide a protective niche for systemic ZIKV spread and a viral reservoir localized in the normally protective blood-brain barrier. Consistent with the spread of ZIKV into neuronal compartments, ZIKV was released basolaterally from hBMECs. Our findings define hBMEC responses that contribute to persistent ZIKV infection and potential targets for clearing ZIKV infections from hBMECs. These results further suggest roles for additional ZIKV-infected ECs to facilitate viral spread and persistence in the protected placental, retinal, and testicular compartments.
Keywords: IFN-β regulation; ISG15 induction; Zika virus; basolateral release; cell survival; chemokine CCL5; human brain endothelial cells; innate immune regulation; persistent infection; transcriptome analysis.
Copyright © 2017 Mladinich et al.
Figures


Similar articles
-
Powassan Viruses Spread Cell to Cell during Direct Isolation from Ixodes Ticks and Persistently Infect Human Brain Endothelial Cells and Pericytes.J Virol. 2022 Jan 12;96(1):e0168221. doi: 10.1128/JVI.01682-21. Epub 2021 Oct 13. J Virol. 2022. PMID: 34643436 Free PMC article.
-
Blockade of Autocrine CCL5 Responses Inhibits Zika Virus Persistence and Spread in Human Brain Microvascular Endothelial Cells.mBio. 2021 Aug 31;12(4):e0196221. doi: 10.1128/mBio.01962-21. Epub 2021 Aug 17. mBio. 2021. PMID: 34399621 Free PMC article.
-
NS5 Sumoylation Directs Nuclear Responses That Permit Zika Virus To Persistently Infect Human Brain Microvascular Endothelial Cells.J Virol. 2020 Sep 15;94(19):e01086-20. doi: 10.1128/JVI.01086-20. Print 2020 Sep 15. J Virol. 2020. PMID: 32699085 Free PMC article.
-
Exploring Zika Virus Impact on Endothelial Permeability: Insights into Transcytosis Mechanisms and Vascular Leakage.Viruses. 2024 Apr 18;16(4):629. doi: 10.3390/v16040629. Viruses. 2024. PMID: 38675970 Free PMC article. Review.
-
Zika Virus Transmission Through Blood Tissue Barriers.Front Microbiol. 2019 Jul 4;10:1465. doi: 10.3389/fmicb.2019.01465. eCollection 2019. Front Microbiol. 2019. PMID: 31333605 Free PMC article. Review.
Cited by
-
Immune response and blood-brain barrier dysfunction during viral neuroinvasion.Innate Immun. 2021 Feb;27(2):109-117. doi: 10.1177/1753425920954281. Epub 2020 Sep 9. Innate Immun. 2021. PMID: 32903111 Free PMC article. Review.
-
Host-microbe interactions at the blood-brain barrier through the lens of induced pluripotent stem cell-derived brain-like endothelial cells.mBio. 2024 Feb 14;15(2):e0286223. doi: 10.1128/mbio.02862-23. Epub 2024 Jan 9. mBio. 2024. PMID: 38193670 Free PMC article. Review.
-
Zika virus enhances monocyte adhesion and transmigration favoring viral dissemination to neural cells.Nat Commun. 2019 Sep 27;10(1):4430. doi: 10.1038/s41467-019-12408-x. Nat Commun. 2019. PMID: 31562326 Free PMC article.
-
A clinical and histopathological study of malformations observed in fetuses infected by the Zika virus.Brain Pathol. 2019 Jan;29(1):114-125. doi: 10.1111/bpa.12644. Epub 2018 Oct 5. Brain Pathol. 2019. PMID: 30020561 Free PMC article.
-
Powassan Viruses Spread Cell to Cell during Direct Isolation from Ixodes Ticks and Persistently Infect Human Brain Endothelial Cells and Pericytes.J Virol. 2022 Jan 12;96(1):e0168221. doi: 10.1128/JVI.01682-21. Epub 2021 Oct 13. J Virol. 2022. PMID: 34643436 Free PMC article.
References
-
- Brasil P, Pereira JP Jr, Moreira ME, Ribeiro Nogueira RM, Damasceno L, Wakimoto M, Rabello RS, Valderramos SG, Halai UA, Salles TS, Zin AA, Horovitz D, Daltro P, Boechat M, Raja Gabaglia CR, Carvalho de Sequeira P, Pilotto JH, Medialdea-Carrera R, Cotrim da Cunha D, Abreu de Carvalho LM, Pone M, Machado Siqueira A, Calvet GA, Rodrigues Baião AE, Neves ES, Nassar de Carvalho PR, Hasue RH, Marschik PB, Einspieler C, Janzen C, Cherry JD, Bispo de Filippis AM, Nielsen-Saines K. 2016. Zika virus infection in pregnant women in Rio de Janeiro. N Engl J Med 375:2321–2334. doi:10.1056/NEJMoa1602412. - DOI - PMC - PubMed
-
- Calvet G, Aguiar RS, Melo AS, Sampaio SA, de Filippis I, Fabri A, Araujo ES, de Sequeira PC, de Mendonça MC, de Oliveira L, Tschoeke DA, Schrago CG, Thompson FL, Brasil P, Dos Santos FB, Nogueira RM, Tanuri A, de Filippis AM. 2016. Detection and sequencing of Zika virus from amniotic fluid of fetuses with microcephaly in Brazil: a case study. Lancet Infect Dis 16:653–660. doi:10.1016/S1473-3099(16)00095-5. - DOI - PubMed
-
- Faria NR, Azevedo Rdo S, Kraemer MU, Souza R, Cunha MS, Hill SC, Theze J, Bonsall MB, Bowden TA, Rissanen I, Rocco IM, Nogueira JS, Maeda AY, Vasami FG, Macedo FL, Suzuki A, Rodrigues SG, Cruz AC, Nunes BT, Medeiros DB, Rodrigues DS, Nunes Queiroz AL, da Silva EV, Henriques DF, Travassos da Rosa ES, de Oliveira CS, Martins LC, Vasconcelos HB, Casseb LM, Simith Dde B, Messina JP, Abade L, Lourenco J, Alcantara LC, de Lima MM, Giovanetti M, Hay SI, de Oliveira RS, Lemos Pda S, de Oliveira LF, de Lima CP, da Silva SP, de Vasconcelos JM, Franco L, Cardoso JF, Vianez JL Jr, Mir D, Bello G, Delatorre E, et al. . 2016. Zika virus in the Americas: Early epidemiological and genetic findings. Science 352:345–349. doi:10.1126/science.aaf5036. - DOI - PMC - PubMed
-
- França GV, Schuler-Faccini L, Oliveira WK, Henriques CM, Carmo EH, Pedi VD, Nunes ML, Castro MC, Serruya S, Silveira MF, Barros FC, Victora CG. 2016. Congenital Zika virus syndrome in Brazil: a case series of the first 1501 livebirths with complete investigation. Lancet 388:891–897. doi:10.1016/S0140-6736(16)30902-3. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
