Loss of mouse cardiomyocyte talin-1 and talin-2 leads to β-1 integrin reduction, costameric instability, and dilated cardiomyopathy
- PMID: 28698364
- PMCID: PMC5544289
- DOI: 10.1073/pnas.1701416114
Loss of mouse cardiomyocyte talin-1 and talin-2 leads to β-1 integrin reduction, costameric instability, and dilated cardiomyopathy
Abstract
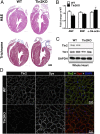
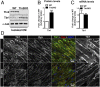
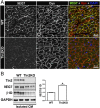
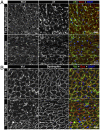
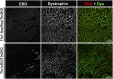
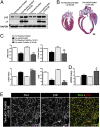
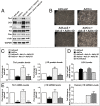
Continuous contraction-relaxation cycles of the heart require strong and stable connections of cardiac myocytes (CMs) with the extracellular matrix (ECM) to preserve sarcolemmal integrity. CM attachment to the ECM is mediated by integrin complexes localized at the muscle adhesion sites termed costameres. The ubiquitously expressed cytoskeletal protein talin (Tln) is a component of muscle costameres that links integrins ultimately to the sarcomere. There are two talin genes, Tln1 and Tln2. Here, we tested the function of these two Tln forms in myocardium where Tln2 is the dominant isoform in postnatal CMs. Surprisingly, global deletion of Tln2 in mice caused no structural or functional changes in heart, presumably because CM Tln1 became up-regulated. Tln2 loss increased integrin activation, although levels of the muscle-specific β1D-integrin isoform were reduced by 50%. With this result, we produced mice that had simultaneous loss of both CM Tln1 and Tln2 and found that cardiac dysfunction occurred by 4 wk with 100% mortality by 6 mo. β1D integrin and other costameric proteins were lost from the CMs, and membrane integrity was compromised. Given that integrin protein reduction occurred with Tln loss, rescue of the phenotype was attempted through transgenic integrin overexpression, but this could not restore WT CM integrin levels nor improve heart function. Our results show that CM Tln2 is essential for proper β1D-integrin expression and that Tln1 can substitute for Tln2 in preserving heart function, but that loss of all Tln forms from the heart-muscle cell leads to myocyte instability and a dilated cardiomyopathy.
Keywords: cardiomyopathy; costameres; heart; integrins; talin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Bloch RJ, et al. Costameres: Repeating structures at the sarcolemma of skeletal muscle. Clin Orthop Relat Res. 2002;(403) Suppl:S203–S210. - PubMed
-
- Bloch RJ, Gonzalez-Serratos H. Lateral force transmission across costameres in skeletal muscle. Exerc Sport Sci Rev. 2003;31:73–78. - PubMed
-
- Hynes RO. Integrins: Bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

