Impact of Autophagy in Oncolytic Adenoviral Therapy for Cancer
- PMID: 28698504
- PMCID: PMC5535969
- DOI: 10.3390/ijms18071479
Impact of Autophagy in Oncolytic Adenoviral Therapy for Cancer
Abstract
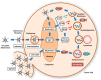
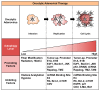
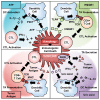
Oncolytic virotherapy has recently emerged as a promising strategy for inducing tumor-specific cell death. Adenoviruses are widely and frequently used in oncolytic virotherapy. The mechanism of oncolytic adenovirus-mediated tumor suppression involves virus-induced activation of the autophagic machinery in tumor cells. Autophagy is a cytoprotective process that produces energy via lysosomal degradation of intracellular components as a physiologic response to various stresses, including hypoxia, nutrient deprivation, and disruption of growth signaling. However, infection with oncolytic adenoviruses induces autophagy and subsequent death of tumor cells rather than enhancing their survival. In this review, we summarize the beneficial role of autophagy in oncolytic adenoviral therapy, including the roles of infection, replication, and cell lysis. Numerous factors are involved in the promotion and inhibition of oncolytic adenovirus-mediated autophagy. Furthermore, recent evidence has shown that oncolytic adenoviruses induce autophagy-related immunogenic cell death (ICD), which enhances the antitumor immune response by inducing the activation of danger signal molecules and thus represents a novel cancer immunotherapy. Understanding the precise role of oncolytic adenovirus-induced autophagy and ICD could enhance the therapeutic potential of oncolytic adenoviral therapy for treating various cancers.
Keywords: autophagy; immunogenic cell death; oncolytic adenovirus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Oncolytic adenovirus-induced autophagy: tumor-suppressive effect and molecular basis.Acta Med Okayama. 2013;67(6):333-42. doi: 10.18926/AMO/52006. Acta Med Okayama. 2013. PMID: 24356717 Review.
-
Crosstalk between oncolytic viruses and autophagy in cancer therapy.Biomed Pharmacother. 2021 Feb;134:110932. doi: 10.1016/j.biopha.2020.110932. Epub 2020 Dec 25. Biomed Pharmacother. 2021. PMID: 33370632 Review.
-
Oncolytic adenoviruses for the treatment of brain tumors.Curr Opin Mol Ther. 2010 Oct;12(5):530-7. Curr Opin Mol Ther. 2010. PMID: 20886384 Review.
-
Oncolytic adenovirus with temozolomide induces autophagy and antitumor immune responses in cancer patients.Mol Ther. 2013 Jun;21(6):1212-23. doi: 10.1038/mt.2013.51. Epub 2013 Apr 2. Mol Ther. 2013. PMID: 23546299 Free PMC article.
-
A novel oncolytic adenovirus targeting Wnt signaling effectively inhibits cancer-stem like cell growth via metastasis, apoptosis and autophagy in HCC models.Biochem Biophys Res Commun. 2017 Sep 16;491(2):469-477. doi: 10.1016/j.bbrc.2017.07.041. Epub 2017 Jul 8. Biochem Biophys Res Commun. 2017. PMID: 28698142
Cited by
-
Metabolome-Driven Regulation of Adenovirus-Induced Cell Death.Int J Mol Sci. 2021 Jan 5;22(1):464. doi: 10.3390/ijms22010464. Int J Mol Sci. 2021. PMID: 33466472 Free PMC article. Review.
-
Telomerase-specific oncolytic immunotherapy for promoting efficacy of PD-1 blockade in osteosarcoma.Cancer Immunol Immunother. 2021 May;70(5):1405-1417. doi: 10.1007/s00262-020-02774-7. Epub 2020 Nov 5. Cancer Immunol Immunother. 2021. PMID: 33151368 Free PMC article.
-
Molecular modulation of autophagy: New venture to target resistant cancer stem cells.World J Stem Cells. 2020 May 26;12(5):303-322. doi: 10.4252/wjsc.v12.i5.303. World J Stem Cells. 2020. PMID: 32547680 Free PMC article. Review.
-
Oncolytic Myxoma virus Increases Autophagy in Multiple Myeloma.Turk J Haematol. 2024 Mar 1;41(1):16-25. doi: 10.4274/tjh.galenos.2024.2023.0403. Epub 2024 Jan 23. Turk J Haematol. 2024. PMID: 38258554 Free PMC article.
-
Autophagy and cancer stem cells: molecular mechanisms and therapeutic applications.Cell Death Differ. 2019 Mar;26(4):690-702. doi: 10.1038/s41418-019-0292-y. Epub 2019 Feb 6. Cell Death Differ. 2019. PMID: 30728463 Free PMC article. Review.
References
-
- Wirth T., Zender L., Schulte B., Mundt B., Plentz R., Rudolph K.L., Manns M., Kubicka S., Kuhnel F. A telomerase-dependent conditionally replicating adenovirus for selective treatment of cancer. Cancer Res. 2003;63:3181–3188. - PubMed
-
- Lanson N.A., Jr., Friedlander P.L., Schwarzenberger P., Kolls J.K., Wang G. Replication of an adenoviral vector controlled by the human telomerase reverse transcriptase promoter causes tumor-selective tumor lysis. Cancer Res. 2003;63:7936–7941. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

