Microfluidic Platform for the Long-Term On-Chip Cultivation of Mammalian Cells for Lab-On-A-Chip Applications
- PMID: 28698531
- PMCID: PMC5539486
- DOI: 10.3390/s17071603
Microfluidic Platform for the Long-Term On-Chip Cultivation of Mammalian Cells for Lab-On-A-Chip Applications
Abstract
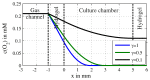
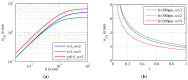
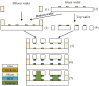
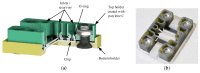
Lab-on-a-Chip (LoC) applications for the long-term analysis of mammalian cells are still very rare due to the lack of convenient cell cultivation devices. The difficulties are the integration of suitable supply structures, the need of expensive equipment like an incubator and sophisticated pumps as well as the choice of material. The presented device is made out of hard, but non-cytotoxic materials (silicon and glass) and contains two vertical arranged membranes out of hydrogel. The porous membranes are used to separate the culture chamber from two supply channels for gases and nutrients. The cells are fed continuously by diffusion through the membranes without the need of an incubator and low requirements on the supply of medium to the assembly. The diffusion of oxygen is modelled in order to find the optimal dimensions of the chamber. The chip is connected via 3D-printed holders to the macroscopic world. The holders are coated with Parlyene C to ensure that only biocompatible materials are in contact with the culture medium. The experiments with MDCK-cells show the successful seeding inside the chip, culturing and passaging. Consequently, the presented platform is a step towards Lab-on-a-Chip applications that require long-term cultivation of mammalian cells.
Keywords: Lab-on-a-Chip; MDCK; cell cultivation; diffusion model; hydrogel; parylene.
Conflict of interest statement
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Figures



References
-
- Schurink B., Luttge R. Hydrogel/poly-dimethylsiloxane hybrid bioreactor facilitating 3D cell culturing. J. Vac. Sci. Technol. B Nanotechnol. Microelectron. Mater. Process. Meas. Phenom. 2013;31:06F903. doi: 10.1116/1.4831762. - DOI
-
- Yu T., Guo Z., Fan H., Song J., Liu Y., Gao Z., Wang Q. Cancer-associated fibroblasts promote non-small cell lung cancer cell invasion by upregulation of glucose-regulated protein 78 (GRP78) expression in an integrated bionic microfluidic device. Oncotarget. 2016;7:25593. doi: 10.18632/oncotarget.8232. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

