Laminin-511 and -521-based matrices for efficient ex vivo-expansion of human limbal epithelial progenitor cells
- PMID: 28698551
- PMCID: PMC5506065
- DOI: 10.1038/s41598-017-04916-x
Laminin-511 and -521-based matrices for efficient ex vivo-expansion of human limbal epithelial progenitor cells
Abstract
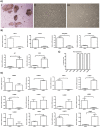
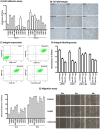
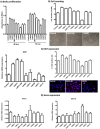
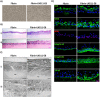
Optimization of culture conditions for human limbal epithelial stem/progenitor cells (LEPC) that incorporate the in vivo cell-matrix interactions are essential to enhance LEPC ex vivo-expansion and transplantation efficiency. Here, we investigate the efficacy of laminin (LN) isoforms preferentially expressed in the limbal niche as culture matrices for epithelial tissue engineering. Analyses of expression patterns of LN chains in the human limbal niche provided evidence for enrichment of LN-α2, -α3, -α5, -β1, -β2, -β3, -γ1, -γ2 and -γ3 chains in the limbal basement membrane, with LN-α5 representing a signature component specifically produced by epithelial progenitor cells. Recombinant human LN-521 and LN-511 significantly enhanced in vitro LEPC adhesion, migration and proliferation compared to other isoforms, and maintained phenotype stability. The bioactive LN-511-E8 fragment carrying only C-terminal domains showed similar efficacy as full-length LN-511. Functional blocking of α3β1 and α6β1 integrins suppressed adhesion of LEPC to LN-511/521-coated surfaces. Cultivation of LEPC on fibrin-based hydrogels incorporating LN-511-E8 resulted in firm integrin-mediated adhesion to the scaffold and well-stratified epithelial constructs, with maintenance of a progenitor cell phenotype in their (supra)basal layers. Thus, the incorporation of chemically defined LN-511-E8 into biosynthetic scaffolds represents a promising approach for xeno-free corneal epithelial tissue engineering for ocular surface reconstruction.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Laminin-511-E8 promotes efficient in vitro expansion of human limbal melanocytes.Sci Rep. 2020 Jul 6;10(1):11074. doi: 10.1038/s41598-020-68120-0. Sci Rep. 2020. PMID: 32632213 Free PMC article.
-
Characterization of extracellular matrix components in the limbal epithelial stem cell compartment.Exp Eye Res. 2007 Dec;85(6):845-60. doi: 10.1016/j.exer.2007.08.020. Epub 2007 Sep 2. Exp Eye Res. 2007. PMID: 17927980
-
Isolation and ex vivo Expansion of Human Limbal Epithelial Progenitor Cells.Bio Protoc. 2020 Sep 20;10(18):e3754. doi: 10.21769/BioProtoc.3754. eCollection 2020 Sep 20. Bio Protoc. 2020. PMID: 33659413 Free PMC article.
-
Characterization, isolation, expansion and clinical therapy of human corneal epithelial stem/progenitor cells.J Stem Cells. 2014;9(2):79-91. J Stem Cells. 2014. PMID: 25158157 Review.
-
Identification and characterization of limbal stem cells.Exp Eye Res. 2005 Sep;81(3):247-64. doi: 10.1016/j.exer.2005.02.016. Exp Eye Res. 2005. PMID: 16051216 Review.
Cited by
-
Integrin: Basement membrane adhesion by corneal epithelial and endothelial cells.Exp Eye Res. 2020 Sep;198:108138. doi: 10.1016/j.exer.2020.108138. Epub 2020 Jul 23. Exp Eye Res. 2020. PMID: 32712184 Free PMC article. Review.
-
Squishy matters - Corneal mechanobiology in health and disease.Prog Retin Eye Res. 2024 Mar;99:101234. doi: 10.1016/j.preteyeres.2023.101234. Epub 2024 Jan 2. Prog Retin Eye Res. 2024. PMID: 38176611 Free PMC article. Review.
-
A decellularized human corneal scaffold for anterior corneal surface reconstruction.Sci Rep. 2021 Feb 4;11(1):2992. doi: 10.1038/s41598-021-82678-3. Sci Rep. 2021. PMID: 33542377 Free PMC article.
-
Regenerating human epithelia with cultured stem cells: feeder cells, organoids and beyond.EMBO Mol Med. 2018 Feb;10(2):139-150. doi: 10.15252/emmm.201708213. EMBO Mol Med. 2018. PMID: 29288165 Free PMC article. Review.
-
Laminin-511-E8 promotes efficient in vitro expansion of human limbal melanocytes.Sci Rep. 2020 Jul 6;10(1):11074. doi: 10.1038/s41598-020-68120-0. Sci Rep. 2020. PMID: 32632213 Free PMC article.
References
-
- Sangwan VS, Tseng SC. New perspectives in ocular surface disorders. An integrated approach for diagnosis and management. Indian J Ophthalmol. 2001;49(3):153–168. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

