Evaluation of Osteogenesis and Angiogenesis of Icariin in Local Controlled Release and Systemic Delivery for Calvarial Defect in Ovariectomized Rats
- PMID: 28698566
- PMCID: PMC5505963
- DOI: 10.1038/s41598-017-05392-z
Evaluation of Osteogenesis and Angiogenesis of Icariin in Local Controlled Release and Systemic Delivery for Calvarial Defect in Ovariectomized Rats
Abstract
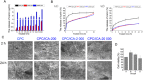
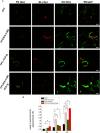
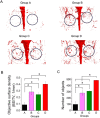
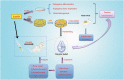
Typically, bone regenerative medicine is applied to repair bone defects in patients with osteoporosis. Meanwhile, there is an urgent need to develop safe and cheap drugs that induce bone formation. Icariin, which is reported to promote the osteogenesis of stem cells in vitro, is the main active component of Herba Epimedii. However, whether icariin could repair bone defects caused by osteoporosis remains unknown. In this study, an osteoporosis model in rats was established by an ovariectomy first, and then, the osteogenic and angiogenic differentiation of bone mesenchymal stem cells (BMSCs) treated with icariin was evaluated. Furthermore, calcium phosphate cement (CPC) scaffolds loaded with icariin were constructed and then implanted into nude mice to determine the optimal construction. To evaluate its osteogenic and angiogenic ability in vivo, this construction was applied to calvarial defect of the ovariectomized (OVX) rats accompanied with an icariin gavage. This demonstrated that icariin could up-regulate the expression of osteogenic and angiogenic genes in BMSCs. Meanwhile, osteoclast formation was inhibited. Moreover, CPC could act as a suitable icariin delivery system for repairing bone defects by enhancing osteogenesis and angiogenesis, while the systemic administration of icariin has an antiosteoporotic effect that promotes bone defect repair.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

