Dopamine 2 Receptor Activation Entrains Circadian Clocks in Mouse Retinal Pigment Epithelium
- PMID: 28698578
- PMCID: PMC5505969
- DOI: 10.1038/s41598-017-05394-x
Dopamine 2 Receptor Activation Entrains Circadian Clocks in Mouse Retinal Pigment Epithelium
Abstract
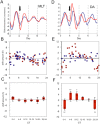
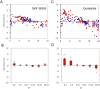
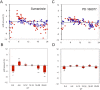
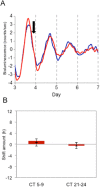
Many of the physiological, cellular, and molecular rhythms that are present within the eye are under the control of circadian clocks. Experimental evidence suggests that the retinal circadian clock, or its output signals (e.g., dopamine and melatonin), may contribute to eye disease and pathology. We recently developed a retinal pigment ephithelium (RPE)-choroid preparation to monitor the circadian clock using PERIOD2 (PER2)::LUC knock-in mouse. In this study we report that dopamine, but not melatonin, is responsible for entrainment of the PER2::LUC bioluminescence rhythm in mouse RPE-choroid. Dopamine induced phase-advances of the PER2::LUC bioluminescence rhythm during the subjective day and phase-delays in the late subjective night. We found that dopamine acts exclusively through Dopamine 2 Receptors to entrain the circadian rhythm in PER2::LUC bioluminescence. Finallly, we found that DA-induced expression of core circadian clock genes Period1 and Period2 accompanied both phase advances and phase delays of the RPE-choroid clock, thus suggesting that - as in other tissues - the rapid induction of these circadian clock genes drives the resetting process. Since the RPE cells persist for the entire lifespan of an organism, we believe that RPE-choroid preparation may represent a new and unique tool to study the effects of circadian disruption during aging.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

