Polyglutamine expansion affects huntingtin conformation in multiple Huntington's disease models
- PMID: 28698602
- PMCID: PMC5505970
- DOI: 10.1038/s41598-017-05336-7
Polyglutamine expansion affects huntingtin conformation in multiple Huntington's disease models
Abstract
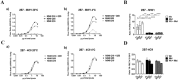
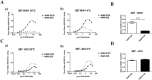
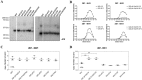
Conformational changes in disease-associated or mutant proteins represent a key pathological aspect of Huntington's disease (HD) and other protein misfolding diseases. Using immunoassays and biophysical approaches, we and others have recently reported that polyglutamine expansion in purified or recombinantly expressed huntingtin (HTT) proteins affects their conformational properties in a manner dependent on both polyglutamine repeat length and temperature but independent of HTT protein fragment length. These findings are consistent with the HD mutation affecting structural aspects of the amino-terminal region of the protein, and support the concept that modulating mutant HTT conformation might provide novel therapeutic and diagnostic opportunities. We now report that the same conformational TR-FRET based immunoassay detects polyglutamine- and temperature-dependent changes on the endogenously expressed HTT protein in peripheral tissues and post-mortem HD brain tissue, as well as in tissues from HD animal models. We also find that these temperature- and polyglutamine-dependent conformational changes are sensitive to bona-fide phosphorylation on S13 and S16 within the N17 domain of HTT. These findings provide key clinical and preclinical relevance to the conformational immunoassay, and provide supportive evidence for its application in the development of therapeutics aimed at correcting the conformation of polyglutamine-expanded proteins as well as the pharmacodynamics readouts to monitor their efficacy in preclinical models and in HD patients.
Conflict of interest statement
The authors declare that they have no competing interests. There are no patents, products in development, or marketed products to declare.
Figures

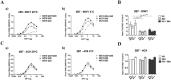




Similar articles
-
Polyglutamine- and temperature-dependent conformational rigidity in mutant huntingtin revealed by immunoassays and circular dichroism spectroscopy.PLoS One. 2014 Dec 2;9(12):e112262. doi: 10.1371/journal.pone.0112262. eCollection 2014. PLoS One. 2014. PMID: 25464275 Free PMC article.
-
Phosphorylation of huntingtin at residue T3 is decreased in Huntington's disease and modulates mutant huntingtin protein conformation.Proc Natl Acad Sci U S A. 2017 Dec 12;114(50):E10809-E10818. doi: 10.1073/pnas.1705372114. Epub 2017 Nov 21. Proc Natl Acad Sci U S A. 2017. PMID: 29162692 Free PMC article.
-
Analysis of mutant and total huntingtin expression in Huntington's disease murine models.Sci Rep. 2020 Dec 17;10(1):22137. doi: 10.1038/s41598-020-78790-5. Sci Rep. 2020. PMID: 33335120 Free PMC article.
-
Huntingtin and Its Partner Huntingtin-Associated Protein 40: Structural and Functional Considerations in Health and Disease.J Huntingtons Dis. 2022;11(3):227-242. doi: 10.3233/JHD-220543. J Huntingtons Dis. 2022. PMID: 35871360 Free PMC article. Review.
-
Degradation of misfolded proteins by autophagy: is it a strategy for Huntington's disease treatment?J Huntingtons Dis. 2013;2(2):149-57. doi: 10.3233/JHD-130052. J Huntingtons Dis. 2013. PMID: 25063512 Review.
Cited by
-
Site-Specific Phosphorylation of Huntingtin Exon 1 Recombinant Proteins Enabled by the Discovery of Novel Kinases.Chembiochem. 2021 Jan 5;22(1):217-231. doi: 10.1002/cbic.202000508. Epub 2020 Oct 13. Chembiochem. 2021. PMID: 32805086 Free PMC article.
-
Docosahexaenoic Acid (DHA) Supplementation in a Triglyceride Form Prevents from Polyglutamine-Induced Dysfunctions in Caenorhabditis elegans.Int J Mol Sci. 2024 Nov 23;25(23):12594. doi: 10.3390/ijms252312594. Int J Mol Sci. 2024. PMID: 39684306 Free PMC article.
-
Unravelling the Helianthus tuberosus L. (Jerusalem Artichoke, Kiku-Imo) Tuber Proteome by Label-Free Quantitative Proteomics.Molecules. 2022 Feb 7;27(3):1111. doi: 10.3390/molecules27031111. Molecules. 2022. PMID: 35164374 Free PMC article.
-
Therapeutic approaches targeting aging and cellular senescence in Huntington's disease.CNS Neurosci Ther. 2024 Oct;30(10):e70053. doi: 10.1111/cns.70053. CNS Neurosci Ther. 2024. PMID: 39428700 Free PMC article. Review.
-
IKBKB reduces huntingtin aggregation by phosphorylating serine 13 via a non-canonical IKK pathway.Life Sci Alliance. 2023 Aug 8;6(10):e202302006. doi: 10.26508/lsa.202302006. Print 2023 Oct. Life Sci Alliance. 2023. PMID: 37553253 Free PMC article.
References
-
- A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. The Huntington’s Disease Collaborative Research Group. Cell72, 971–83 (1993). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

