Naringenin Impairs Two-Pore Channel 2 Activity And Inhibits VEGF-Induced Angiogenesis
- PMID: 28698624
- PMCID: PMC5505983
- DOI: 10.1038/s41598-017-04974-1
Naringenin Impairs Two-Pore Channel 2 Activity And Inhibits VEGF-Induced Angiogenesis
Abstract
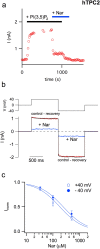
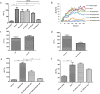
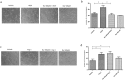
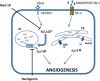
Our research introduces the natural flavonoid naringenin as a novel inhibitor of an emerging class of intracellular channels, Two-Pore Channel 2 (TPC2), as shown by electrophysiological evidence in a heterologous system, i.e. Arabidopsis vacuoles lacking endogenous TPCs. In view of the control exerted by TPC2 on intracellular calcium signaling, we demonstrated that naringenin dampens intracellular calcium responses of human endothelial cells stimulated with VEGF, histamine or NAADP-AM, but not with ATP or Angiopoietin-1 (negative controls). The ability of naringenin to impair TPC2-dependent biological activities was further explored in an established in vivo model, in which VEGF-containing matrigel plugs implanted in mice failed to be vascularized in the presence of naringenin. Overall, the present data suggest that naringenin inhibition of TPC2 activity and the observed inhibition of angiogenic response to VEGF are linked by impaired intracellular calcium signaling. TPC2 inhibition is emerging as a key therapeutic step in a range of important pathological conditions including the progression and metastatic potential of melanoma, Parkinson's disease, and Ebola virus infection. The identification of naringenin as an inhibitor of TPC2-mediated signaling provides a novel and potentially relevant tool for the advancement of this field of research.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

