Intercellular adhesion molecule-1 augments myoblast adhesion and fusion through homophilic trans-interactions
- PMID: 28698658
- PMCID: PMC5506053
- DOI: 10.1038/s41598-017-05283-3
Intercellular adhesion molecule-1 augments myoblast adhesion and fusion through homophilic trans-interactions
Abstract
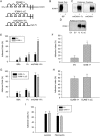
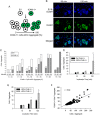
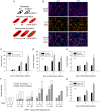
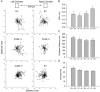
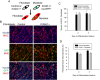
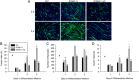
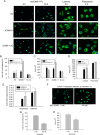
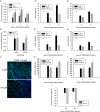
The overall objective of the study was to identify mechanisms through which intercellular adhesion molecule-1 (ICAM-1) augments the adhesive and fusogenic properties of myogenic cells. Hypotheses were tested using cultured myoblasts and fibroblasts, which do not constitutively express ICAM-1, and myoblasts and fibroblasts forced to express full length ICAM-1 or a truncated form lacking the cytoplasmic domain of ICAM-1. ICAM-1 mediated myoblast adhesion and fusion were quantified using novel assays and cell mixing experiments. We report that ICAM-1 augments myoblast adhesion to myoblasts and myotubes through homophilic trans-interactions. Such adhesive interactions enhanced levels of active Rac in adherent and fusing myoblasts, as well as triggered lamellipodia, spreading, and fusion of myoblasts through the signaling function of the cytoplasmic domain of ICAM-1. Rac inhibition negated ICAM-1 mediated lamellipodia, spreading, and fusion of myoblasts. The fusogenic property of ICAM-1-ICAM-1 interactions was restricted to myogenic cells, as forced expression of ICAM-1 by fibroblasts did not augment their fusion to ICAM-1+ myoblasts/myotubes. We conclude that ICAM-1 augments myoblast adhesion and fusion through its ability to self-associate and initiate Rac-mediated remodeling of the actin cytoskeleton.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Pavlath, G. K. Current Progress Towards Understanding Mechanisms of Myoblast Fusion in Mammals. (Springer-Verlag Berlin, Heidelberger Platz 3, D-14197 Berlin, Germany, 2011).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

