Modelling person-to-person transmission in an Enterovirus A71 orally infected hamster model of hand-foot-and-mouth disease and encephalomyelitis
- PMID: 28698666
- PMCID: PMC5567166
- DOI: 10.1038/emi.2017.49
Modelling person-to-person transmission in an Enterovirus A71 orally infected hamster model of hand-foot-and-mouth disease and encephalomyelitis
Abstract
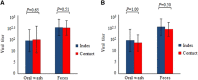
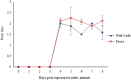
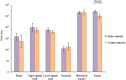
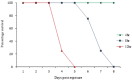
Enterovirus A71 (EV-A71) causes hand-foot-and-mouth disease (HFMD), which may be complicated by fatal encephalomyelitis. Although fecal-oral or oral-oral routes are important in person-to-person transmission, how viral shedding and exposure may predispose individuals to infection remains unknown. We investigated person-to-person transmission by using a model of HFMD and encephalomyelitis based on EV-A71 oral infection of 2-week-old hamsters. Animals (index animals) infected with 104 50% cell culture infective doses of virus uniformly developed severe disease four days post-infection (dpi), whereas littermate contacts developed severe disease after six to seven days of exposure to index animals. Virus was detected in oral washes and feces at 3-4 dpi in index animals and at three to eight days after exposure to index animals in littermate contact animals. In a second experiment, non-littermate contact animals exposed for 8 or 12 h to index animals developed the disease six and four days post-exposure, respectively. Tissues from killed index and contact animals, studied by light microscopy, immunohistochemistry and in situ hybridization, exhibited mild inflammatory lesions and/or viral antigens/RNA in the squamous epithelia of the oral cavity, tongue, paws, skin, esophagus, gastric epithelium, salivary glands, lacrimal glands, central nervous system neurons, muscles (skeletal, cardiac and smooth muscles) and liver. Orally shed viruses were probably derived from infected oral mucosa and salivary glands, whereas fecal viruses may have derived from these sites as well as from esophageal and gastric epithelia. Asymptomatic seroconversion in exposed mother hamsters was demonstrated. Our hamster model should be useful in studying person-to-person EV-A71 transmission and how drugs and vaccines may interrupt transmission.
Figures
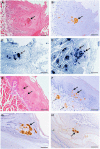
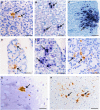
References
-
- Knipe DM, Howley PM. Fields Virology. In: Griffin DE et al (eds). Enteroviruses: Polioviruses, Coxsackieviruses, Echoviruses, and Newer Enteroviruses. 4th ed. USA: Lippincott Williams & Wilkins. 2001, 723–775.
-
- Ho M, Chen ER, Hsu KH et al. An epidemic of enterovirus 71 infection in Taiwan. N Engl J Med 1999; 341: 929–935. - PubMed
-
- Ooi MH, Wong SC, Podin Y et al. Human enterovirus 71 disease in Sarawak, Malaysia: a prospective clinical, virological, and molecular epidemiological study. Clin Infect Dis 2007; 44: 646–656. - PubMed
-
- Mcminn PC. Enterovirus 71 in the Asia-Pacific region: an emerging cause of acute neurological disease in young children. Neurol J Southeast Asia 2003; 8: 57–63.
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
