Modulatory Effect of Monochromatic Blue Light on Heat Stress Response in Commercial Broilers
- PMID: 28698764
- PMCID: PMC5494062
- DOI: 10.1155/2017/1351945
Modulatory Effect of Monochromatic Blue Light on Heat Stress Response in Commercial Broilers
Abstract
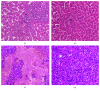
In a novel approach, monochromatic blue light was used to investigate its modulatory effect on heat stress biomarkers in two commercial broiler strains (Ross 308 and Cobb 500). At 21 days old, birds were divided into four groups including one group housed in white light, a second group exposed to blue light, a 3rd group exposed to white light + heat stress, and a 4th group exposed to blue light + heat stress. Heat treatment at 33°C lasted for five h for four successive days. Exposure to blue light during heat stress reduced MDA concentration and enhanced SOD and CAT enzyme activities as well as modulated their gene expression. Blue light also reduced the degenerative changes that occurred in the liver tissue as a result of heat stress. It regulated, though variably, liver HSP70, HSP90, HSF1, and HSF3 gene expression among Ross and Cobb chickens. Moreover, the Cobb strain showed better performance than Ross manifested by a significant reduction of rectal temperature in the case of H + B. Furthermore, a significant linear relationship was found between the lowered rectal temperature and the expression of all HSP genes. Generally, the performance of both strains by most assessed parameters under heat stress is improved when using blue light.
Figures



References
-
- Allen C. D., Macalady A. K., Chenchouni H., et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. Forest Ecology and Management. 2010;259(4):660–684. - PubMed
-
- Akbarian A., Michiels J., Degroote J., Majdeddin M., Golian A., De Smet S. Association between heat stress and oxidative stress in poultry; mitochondrial dysfunction and dietary interventions with phytochemicals. Journal of Animal Science and Biotechnology. 2016;7(37):1–14. doi: 10.1007/s13197-016-2361-2. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

