Targeting the reactive intermediate in polysaccharide monooxygenases
- PMID: 28698982
- PMCID: PMC5613103
- DOI: 10.1007/s00775-017-1480-1
Targeting the reactive intermediate in polysaccharide monooxygenases
Abstract
Lytic polysaccharide monooxygenases (LPMOs) are copper metalloenzymes that can enhance polysaccharide depolymerization through an oxidative mechanism, making them interesting for the production of biofuel from cellulose. However, the details of this activation are unknown; in particular, the nature of the intermediate that attacks the glycoside C-H bond in the polysaccharide is not known, and a number of different species have been suggested. The homolytic bond-dissociation energy (BDE) has often been used as a descriptor for the bond-activation power, especially for inorganic model complexes. We have employed quantum-chemical cluster calculations to estimate the BDE for a number of possible LPMO intermediates to bridge the gap between model complexes and the actual LPMO active site. The calculated BDEs suggest that the reactive intermediate is either a Cu(II)-oxyl, a Cu(III)-oxyl, or a Cu(III)-hydroxide, which indicate that O-O bond breaking occurs before the C-H activation step.
Keywords: Computational chemistry; Density functional theory; Lytic polysaccharide monooxygenase; Reaction mechanism.
Figures



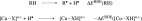


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

