Medication Errors: A Characterisation of Spontaneously Reported Cases in EudraVigilance
- PMID: 28698988
- PMCID: PMC5688193
- DOI: 10.1007/s40264-017-0569-3
Medication Errors: A Characterisation of Spontaneously Reported Cases in EudraVigilance
Erratum in
-
Correction to: Medication Errors: A Characterisation of Spontaneously Reported Cases in EudraVigilance.Drug Saf. 2017 Dec;40(12):1293. doi: 10.1007/s40264-017-0609-z. Drug Saf. 2017. PMID: 29116609 Free PMC article.
-
Correction to: Medication Errors: A Characterisation of Spontaneously Reported Cases in EudraVigilance.Drug Saf. 2018 Dec;41(12):1439-1440. doi: 10.1007/s40264-018-0700-0. Drug Saf. 2018. PMID: 30027420 Free PMC article.
Abstract
Introduction: Medication errors recently became the focus of regulatory guidance in pharmacovigilance to support reporting, evaluation and prevention of medication errors.
Objective: This study aims to characterise spontaneously reported cases of medication errors in EudraVigilance over the period 2002-2015 before the release of EU good practice guidance.
Methods: Case reports were identified through the adverse reaction section where a Medical Dictionary for Regulatory Activities (MedDRA®) term is reported and included in the Standardised MedDRA® Query (SMQ) for medication errors. These case reports were further categorised by MedDRA® terms, geographical region, patient age group and Anatomical Therapeutic Chemical classification system of suspect medicinal product(s).
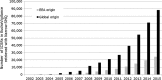
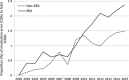
Results: A total of 147,824 case reports were retrieved, 41,355 of which were from the European Economic Area (EEA). Approximately 60% of these case reports were retrieved with the narrow SMQ. The absolute number of medication error case reports and the proportion to the total number of reports in EudraVigilance increased during the study period, with peaks seen around 2005 and 2012 for cases with EEA origin. Fifty-two percent of case reports in which age was provided occurred in adults, 30% in the elderly and 18% in children, with almost half of these in children aged 2 months to 2 years.
Conclusion: Case reports of medication errors in EudraVigilance steadily increased between 2005 and 2015, the reasons for which may be multifactorial, including increased awareness, changes to the MedDRA® terminology and the 2012 EU pharmacovigilance legislation and associated guidance for stakeholders, or a generally increased risk for errors as more medications become available.
Conflict of interest statement
Funding
No sources of funding were used to assist in the preparation of this article.
Conflicts of interest
Victoria Newbould, Steven Le Meur, Thomas Goedecke and Xavier Kurz have no conflicts of interest that are directly relevant to the content of this study.
Disclaimer
The views expressed in this article are the personal views of the authors and may not be understood or quoted as being made on behalf of or reflecting the position of the EMA or one of its committees or working parties.
Figures



Similar articles
-
Disproportionality Analysis and Characterisation of Medication Errors in EudraVigilance: Exploring Findings on Sexes and Age Groups.Drug Saf. 2025 Jan;48(1):59-74. doi: 10.1007/s40264-024-01478-6. Epub 2024 Sep 19. Drug Saf. 2025. PMID: 39300043 Free PMC article.
-
Spontaneous reports of vaccination errors in the European regulatory database EudraVigilance: A descriptive study.Vaccine. 2018 Dec 18;36(52):7956-7964. doi: 10.1016/j.vaccine.2018.11.003. Epub 2018 Nov 8. Vaccine. 2018. PMID: 30416019
-
Medication Errors: New EU Good Practice Guide on Risk Minimisation and Error Prevention.Drug Saf. 2016 Jun;39(6):491-500. doi: 10.1007/s40264-016-0410-4. Drug Saf. 2016. PMID: 26940903 Review.
-
Patient Reporting in the EU: Analysis of EudraVigilance Data.Drug Saf. 2017 Jul;40(7):629-645. doi: 10.1007/s40264-017-0534-1. Drug Saf. 2017. PMID: 28417320
-
Strengthening and rationalizing pharmacovigilance in the EU: where is Europe heading to? A review of the new EU legislation on pharmacovigilance.Drug Saf. 2011 Mar 1;34(3):187-97. doi: 10.2165/11586620-000000000-00000. Drug Saf. 2011. PMID: 21332243 Review.
Cited by
-
Suicidal and accidental drug poisoning mortality among older adults and working-age individuals in Spain between 2000 and 2018.BMC Geriatr. 2022 Feb 10;22(1):114. doi: 10.1186/s12877-022-02806-0. BMC Geriatr. 2022. PMID: 35144558 Free PMC article.
-
Implementation and comparison of two text mining methods with a standard pharmacovigilance method for signal detection of medication errors.BMC Med Inform Decis Mak. 2020 May 24;20(1):94. doi: 10.1186/s12911-020-1097-0. BMC Med Inform Decis Mak. 2020. PMID: 32448248 Free PMC article.
-
Quality of MedDRA® Coding in a Sample of COVID-19 Vaccine Medication Error Data.Drug Saf. 2023 May;46(5):501-507. doi: 10.1007/s40264-023-01294-4. Epub 2023 Apr 23. Drug Saf. 2023. PMID: 37087705 Free PMC article.
-
Dissemination of Direct Healthcare Professional Communications on Medication Errors for Medicinal Products in the EU: An Explorative Study on Relevant Factors.Drug Saf. 2021 Jan;44(1):73-82. doi: 10.1007/s40264-020-00995-4. Drug Saf. 2021. PMID: 33355904 Free PMC article.
-
Methodological approaches for medication error analyses in patient safety and pharmacovigilance reporting systems: a scoping review protocol.BMJ Open. 2022 May 24;12(5):e057764. doi: 10.1136/bmjopen-2021-057764. BMJ Open. 2022. PMID: 35613756 Free PMC article.
References
-
- Union European. Directive 2001/83/EC of the European Parliament and of the Council of 6 November 2001 on the Community code relating to medicinal products for human use. OJ. 2001;44(L311):67–128.
-
- Pharmacovigilance Risk Assessment Committee. Good practice guide on recording, coding, reporting and assessment of medication errors (EMA/762563/2014). http://www.ema.europa.eu/docs/en_GB/document_library/Regulatory_and_proc... (2015). Accessed 15 Jun 2017.
-
- European Medicines Agency. Guideline on good pharmacovigilance practices (GVP) Module VI—management and reporting of adverse reactions to medicinal products (Rev 1) (EMA/873138/2011 Rev 1). http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guidelin... (2014). Accessed 15 Jun 2017.
-
- E2B(R3) Individual Case Safety Report (ICSR). Specification and related files. http://estri.ich.org/e2br3/index.htm. Accessed 19 Jun 2017.
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

