Practical Recommendations for Therapeutic Drug Monitoring of Kinase Inhibitors in Oncology
- PMID: 28699160
- PMCID: PMC5656880
- DOI: 10.1002/cpt.787
Practical Recommendations for Therapeutic Drug Monitoring of Kinase Inhibitors in Oncology
Abstract
Despite the fact that pharmacokinetic exposure of kinase inhibitors (KIs) is highly variable and clear relationships exist between exposure and treatment outcomes, fixed dosing is still standard practice. This review aims to summarize the available clinical pharmacokinetic and pharmacodynamic data into practical guidelines for individualized dosing of KIs through therapeutic drug monitoring (TDM). Additionally, we provide an overview of prospective TDM trials and discuss the future steps needed for further implementation of TDM of KIs.
© 2017 The Authors Clinical Pharmacology & Therapeutics published by Wiley Periodicals, Inc. on behalf of American Society for Clinical Pharmacology and Therapeutics.
Figures
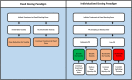
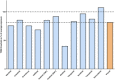
References
-
- Yu, H. et al Practical guidelines for therapeutic drug monitoring of anticancer tyrosine kinase inhibitors: focus on the pharmacokinetic targets. Clin. Pharmacokinet. 53, 305–325 (2014). - PubMed
-
- Food and Drug Administration . Center for Drug Evaluation and Research Alectinib Clinical Pharmacology and Biopharmaceutics Review. <http://www.accessdata.fda.gov/drugsatfda_docs/nda/2015/208434Orig1s000Cl...> (2016).
-
- Food and Drug Administration . Center for Drug Evaluation and Research Ceritinib Clinical Pharmacology and Biopharmaceutics Review. <http://www.accessdata.fda.gov/drugsatfda_docs/nda/2014/205755Orig1s000Cl...> (2014).
-
- Committee for Medicinal Products for Human Use (CHMP) European Medicines Agency Ceritinib European Public Assessment report. 44, (2015).
-
- European Medicines Agency Committee for Medicinal Products For Human Use (CHMP) Crizotinib European Public Assessment Report. <http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_‐_Public_asses...> (2012).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

