Next Generation Hemostatic Materials Based on NHS-Ester Functionalized Poly(2-oxazoline)s
- PMID: 28699748
- PMCID: PMC5558194
- DOI: 10.1021/acs.biomac.7b00683
Next Generation Hemostatic Materials Based on NHS-Ester Functionalized Poly(2-oxazoline)s
Abstract
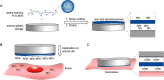
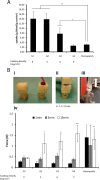
In order to prevent hemorrhage during surgical procedures, a wide range of hemostatic agents have been developed. However, their efficacy is variable; hemostatic devices that use bioactive components to accelerate coagulation are dependent on natural sources, which limits reproducibility. Hybrid devices in which chain-end reactive poly(ethylene glycol) is employed as active component sometimes suffer from irregular cross-linking and dissolution of the polar PEG when blood flow is substantial. Herein, we describe a synthetic, nonbioactive hemostatic product by coating N-hydroxysuccinimide ester (NHS)-functional poly(2-oxazoline)s (POx-NHS) onto gelatin patches, which acts by formation of covalent cross-links between polymer, host blood proteins, gelatin and tissue to seal the wound site and prevent hemorrhage during surgery. We studied different process parameters (including polymer, carrier, and coating technique) in direct comparison with clinical products (Hemopatch and Tachosil) to obtain deeper understanding of this class of hemostatic products. In this work, we successfully prove the hemostatic efficacy of POx-NHS as polymer powders and coated patches both in vitro and in vivo against Hemopatch and Tachosil, demonstrating that POx-NHS are excellent candidate polymers for the development of next generation hemostatic patches.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Janssen P. F.; Brölmann H. A. M.; Huirne J. A. F. Effectiveness of electrothermal bipolar vessel-sealing devices versus other electrothermal and ultrasonic devices for abdominal surgical hemostasis: a systematic review. Surg. Endosc. 2012, 26 (10), 2892–2901. 10.1007/s00464-012-2276-6. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

