Oral paracetamol (acetaminophen) for cancer pain
- PMID: 28700092
- PMCID: PMC6369932
- DOI: 10.1002/14651858.CD012637.pub2
Oral paracetamol (acetaminophen) for cancer pain
Abstract
Background: Pain is a common symptom with cancer, and 30% to 50% of all people with cancer will experience moderate to severe pain that can have a major negative impact on their quality of life. Non-opioid drugs are commonly used to treat mild to moderate cancer pain, and are recommended for this purpose in the WHO cancer pain treatment ladder, either alone or in combination with opioids.A previous Cochrane review that examined the evidence for nonsteroidal anti-inflammatory drugs (NSAIDs) or paracetamol, alone or combined with opioids, for cancer pain was withdrawn in 2015 because it was out of date; the date of the last search was 2005. This review, and another on NSAIDs, updates the evidence.
Objectives: To assess the efficacy of oral paracetamol (acetaminophen) for cancer pain in adults and children, and the adverse events reported during its use in clinical trials.
Search methods: We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, and Embase from inception to March 2017, together with reference lists of retrieved papers and reviews, and two online study registries.
Selection criteria: We included randomised, double-blind, studies of five days' duration or longer, comparing paracetamol alone with placebo, or paracetamol in combination with an opioid compared with the same dose of the opioid alone, for cancer pain of any intensity. Single-blind and open studies were also eligible for inclusion. The minimum study size was 25 participants per treatment arm at the initial randomisation.
Data collection and analysis: Two review authors independently searched for studies, extracted efficacy and adverse event data, and examined issues of study quality and potential bias. We did not carry out any pooled analyses. We assessed the quality of the evidence using GRADE and created a 'Summary of findings' table.
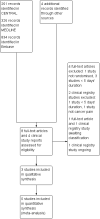
Main results: Three studies in adults satisfied the inclusion criteria, lasting up to one week; 122 participants were randomised initially, and 95 completed treatment. We found no studies in children. One study was parallel-group, and two had a cross-over design. All used paracetamol as an add-on to established treatment with strong opioids (median daily morphine equivalent doses of 60 mg, 70 mg, and 225 mg, with some participants taking several hundred mg of oral morphine equivalents daily). Other non-paracetamol medication included non-steroidal anti-inflammatory drugs (NSAIDs), tricyclic antidepressants, or neuroleptics. All studies were at high risk of bias for incomplete outcome data and small size; none was unequivocally at low risk of bias.None of the studies reported any of our primary outcomes: participants with pain reduction of at least 50%, and at least 30%, from baseline; participants with pain no worse than mild at the end of the treatment period; participants with Patient Global Impression of Change (PGIC) of much improved or very much improved (or equivalent wording). What pain reports there were indicated no difference between paracetamol and placebo when added to another treatment. There was no convincing evidence of paracetamol being different from placebo with regards to quality of life, use of rescue medication, or participant satisfaction or preference. Measures of harm (serious adverse events, other adverse events, and withdrawal due to lack of efficacy) were inconsistently reported and provided no clear evidence of difference.Our GRADE assessment of evidence quality was very low for all outcomes, because studies were at high risk of bias from several sources.
Authors' conclusions: There is no high-quality evidence to support or refute the use of paracetamol alone or in combination with opioids for the first two steps of the three-step WHO cancer pain ladder. It is not clear whether any additional analgesic benefit of paracetamol could be detected in the available studies, in view of the doses of opioids used.
Conflict of interest statement
PW: none known.
SD: none known.
RAM has received grant support from Grünenthal relating to individual patient‐level analyses of trial data regarding tapentadol in osteoarthritis and back pain (2015). He has received honoraria for attending boards with Menarini concerning methods of analgesic trial design (2014), with Novartis (2014) about the design of network meta‐analyses, and RB on understanding pharmacokinetics of drug uptake (2015). He has received honoraria from Omega Pharma (2016) and Futura Pharma (2016) for providing advice on trial and data analysis methods.
EDM: none known.
RFB: none known. RFB is a specialised pain physician and has managed patients with cancer pain.
DBC: none known. DBC is a specialised pain physician and has managed patients with cancer pain.
MM: none known.
BW: none known. BW is a specialist Palliative Medicine Consultant physician and manages patients with advanced life‐threatening illnesses, including cancer.
This review was identified in a 2019 audit as not meeting the current definition of the Cochrane Commercial Sponsorship policy. At the time of its publication it was compliant with the interpretation of the existing policy. As with all reviews, new and updated, at update this review will be revised according to 2020 policy update.
Figures
Update of
References
References to studies included in this review
Axelsson 2003 {published data only}
Cubero 2010 {published data only}
Israel 2010 {published data only}
-
- Israel FJ, Parker G, Charles M, Reymond L. Lack of benefit from paracetamol (acetaminophen) for palliative cancer patients requiring high‐dose strong opioids: a randomized, double‐blind, placebo‐controlled, crossover trial. Journal of Pain and Symptom Management 2010;39(3):548‐54. [DOI: 10.1016/j.jpainsymman.2009.07.008] - DOI - PubMed
References to studies excluded from this review
ACTRN12610000893000 {published data only}
-
- Mitchell G (Principal Investigator). A single patient multiple cross‐over study to determine the efficacy of paracetamol in relieving pain suffered by patients with advanced cancer taking regular opioids. www.anzctr.org.au/ACTRN12610000893000.aspx (accessed 30 March 2017) 2010. [ANZCTR: ACTRN12610000893000] - PubMed
Axelsson 2008 {published data only}
Danninger 1993 {published data only}
-
- Danninger R, Jakse R, Beubler E. Randomized crossover‐comparison of 2 g‐ and 4 g‐doses of paracetamol per day in the case of mild to moderate pain caused by head and neck cancers. European Journal of Pain 1993;14(1):14‐8. [EMBASE: 132642 xx]
JPRN‐UMIN000009292 {published data only}
-
- Ken‐ichi Kaneko (Principle Investigator). Efficacy and safety of acetaminophen in the treatment of patients with head and neck cancer. upload.umin.ac.jp/cgi‐open‐bin/ctr_e/ctr_view.cgi?recptno=R000010522 (accessed 30 March 2017).
Nickles 2016 {published data only}
-
- Nickles J, Mitchell GK, Hardy J, Senior H, Carmont S‐A, Schluter PJ, et al. Single‐patient multiple crossover studies to determine the effectiveness of paracetamol in relieving pain suffered by patients with advanced cancer taking regular opioids: A pilot study. Palliative Medicine 2016;30(8):800‐2. [ANZCTR: ACTRN12609000870257; DOI: 10.1177/0269216316635012] - DOI - PubMed
Stockler 2004 {published data only}
-
- Stockler M, Vardy J, Pillai A, Warr D. Acetaminophen (paracetamol) improves pain and well‐being in people with advanced cancer already receiving a strong opioid regimen: a randomized, double‐blind, placebo‐controlled cross‐over trial. Journal of Clinical Oncology 2004;22(16):3389‐94. [DOI: 10.1200/JCO.2004.09.122] - DOI - PubMed
References to studies awaiting assessment
NCT00152854 {published data only}
-
- Vardy J (Principal Investigator). A randomised, placebo‐controlled, crossover trial of acetaminophen in cancer patients on strong opioids. clinicaltrials.gov/ct2/show/NCT00152854 (accessed 30 March 2017) 2016. [CTG: NCT00152854]
Pacilio 1989 {published data only}
-
- Pacilio G, Pizza C, Vetrano A, Mastrantoni M, Ceccarelli G. A double‐blind study on the effect of flupirtine as compared to paracetamol in the cancer pain. Riforma Medica 1989;104(1‐2):29‐32. [EMBASE: 1989176982]
References to ongoing studies
NCT02706769 {unpublished data only}
-
- University of Edinburgh. A double‐blind randomised parallel group trial of paracetamol versus placebo in conjunction with strong opioids for cancer related pain. clinicaltrials.gov/ct2/show/NCT02706769 (accessed 30 March 2017) 2016.
Additional references
AlBalawi 2013
Altyar 2015
Axelrod 2003
-
- Axelrod J. Journey of a late blooming biochemical neuroscientist. Journal of Biology and Chemistry 2003;278(1):1‐13. - PubMed
Azevedo São Leão Ferreira 2006
Bao 2016
Bateman 2014a
-
- Bateman DN, Carroll R, Pettie J, Yamamoto T, Elamin ME, Peart L, et al. Effect of the UK's revised paracetamol poisoning management guidelines on admissions, adverse reactions, and costs of treatment. British Journal of Clinical Pharmacology 2014;78(3):610‐8. [DOI: 10.1111/bcp.12362] - DOI - PMC - PubMed
Bateman 2014b
Bell 2006
Botting 2000
Breivik 2009
Brok 2009
-
- Brok J, Thorlund K, Wetterslev J, Gluud C. Apparently conclusive meta‐analyses may be inconclusive ‐ trial sequential analysis adjustment of random error risk due to repetitive testing of accumulating data in apparently conclusive neonatal meta‐analyses. International Journal of Epidemiology 2009;38(1):287‐98. [DOI: 10.1093/ije/dyn188] - DOI - PubMed
Brune 2015
-
- Brune K, Renner B, Tiegs G. Acetaminophen/paracetamol: a history of errors, failures and false decisions. European Journal of Pain 2015;19(7):953‐65. [10.1002/ejp.621] - PubMed
Cancer Research UK 2016
-
- Cancer statistics for the UK. www.cancerresearchuk.org/health‐professional/cancer‐statistics (accessed 21 November 2016).
Carlson 2016
Chandrasekharan 2002
-
- Chandrasekharan NV, Dai H, Roos KL, Evanson NK, Tomsik J, Elton TS, et al. COX‐3, a cyclooxygenase‐1 variant inhibited by acetaminophen and other analgesic/antipyretic drugs: cloning, structure and expression. Proceedings of the National Academy of Sciences of the United States of America 2002;99(21):13926‐31. [DOI: 10.1073/pnas.162468699] - DOI - PMC - PubMed
Cooper 2017
CSM 1997
-
- Committee on Safety of Medicines. Medicines Control Agency. Paracetamol and aspirin. Current Problems in Pharmacovigilance 1997;23:9.
Curros‐Criado 2009
Dani 2007
Dart 2000
-
- Dart RC, Kuffner EK, Rumack BH. Treatment of pain or fever with paracetamol (acetaminophen) in the alcoholic patient: a systematic review. American Journal of Medicine 2000;7(2):123‐34. [PUBMED: 11319580] - PubMed
Deandrea 2008
Dechartes 2013
Dechartres 2014
Deng 2012
Derry 2013
Derry 2014
Derry 2017
Dreidi 2016
Drugs.com 2016
-
- Anonymous. Paracetamol. www.drugs.com/international/paracetamol.html (accessed 10 May 2016).
Dworkin 2008
Elbourne 2002
-
- Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. - PubMed
EPOC 2015
-
- Effective Practice, Organisation of Care (EPOC). 23. Worksheets for preparing a Summary of Findings using GRADE. Resources for review authors. epoc.cochrane.org/epoc‐specific‐resources‐review‐authors (accessed 30 November 2016).
Fanelli 2017
FDA 2015
-
- Hertz S. Review of Acetaminophen for the Withdrawn or Removed List. www.fda.gov/downloads/advisorycommittees/committeesmeetingmaterials/drug... May 13 2015 (Accessed May 30 2017):44 et seq.
Flower 1972
-
- Flower RJ, Vane JR. Inhibition of prostaglandin synthetase in brain explains the anti‐pyretic activity of paracetamol (4‐acetamidophenol). Nature 1972;240(5381):410‐1. [PUBMED: 4564318] - PubMed
Gavaghan 2000
-
- Gavaghan DJ, Moore RA, McQuay HJ. An evaluation of homogeneity tests in meta‐analyses in pain using simulations of individual patient data. Pain 2000;85(3):415‐24. - PubMed
Graham 2005
Gulcu 2007
Gulmez 2013
-
- Gulmez SE, Larrey D, Pageaux GP, Lignot S, Lassalle R, Jové J, et al. Transplantation for acute liver failure in patients exposed to NSAIDs or paracetamol (acetaminophen): the multinational case‐population SALT study. Drug Safety 2013;36(2):135‐44. [DOI: 10.1007/s40264-012-0013-7] - DOI - PMC - PubMed
Gulmez 2015
Gunnell 1997
Guyatt 2011
Guyatt 2013a
-
- Guyatt G, Oxman AD, Sultan S, Brozek J, Glasziou P, Alonso‐Coello P, et al. GRADE guidelines: 11. Making an overall rating of confidence in effect estimates for a single outcome and for all outcomes. Journal of Clinical Epidemiology 2013;66:151‐7. [DOI: 10.1016/j.jclinepi.2012.01.006] - DOI - PubMed
Guyatt 2013b
Hadley 2013
Hawkins 2007
-
- Hawkins LC, Edwards JN, Dargan PI. Impact of restricting paracetamol pack sizes on paracetamol poisoning in the United Kingdom: a review of the literature. Drug Safety 2007;30(6):465‐79. [PUBMED: 17536874] - PubMed
Hawton 2001
Hawton 2013
Higgins 2011
-
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hinz 2008
IARC 2012
-
- International Agency for Research on Cancer. GLOBOCAN 2012: Estimated cancer incidence, mortality and prevalence worldwide in 2012. globocan.iarc.fr/Pages/fact_sheets_cancer.aspx (accessed 21 November 2016).
IntHout 2015
Jadad 1995
-
- Jadad AR, Browman GP. The WHO analgesic ladder for cancer pain management. Stepping up the quality of Its evaluation. JAMA 1995;274(23):1870‐3. - PubMed
Liew 2014
Machado 2015
Mazaud‐Guittot 2013
-
- Mazaud‐Guittot S, Nicolas Nicolaz C, Desdoits‐Lethimonier C, Coiffec I, Ben Maamar M, Balaguer P, et al. Paracetamol, aspirin, and indomethacin induce endocrine disturbances in the human fetal testis capable of interfering with testicular descent. Journal of Clinical Endocrinology and Metabolism 2013;98(11):1757‐67. [DOI: 10.1210/jc.2013-2531] - DOI - PubMed
McQuay 1998
-
- McQuay H, Moore A. An Evidence‐based Resource for Pain Relief. Oxford: Oxford University Press, 1998. [ISBN: 0‐19‐263048‐2]
McQuay 2005
McQuay 2007
Mercadante 2013
Mercadante 2014
Mikan 2016
-
- Mikan F, Wada M, Yamada M, Takahashi A, Onishi H, Ishida M, et al. The association between pain and quality of life for patients with cancer in an outpatient clinic, an inpatient oncology ward, and inpatient palliative care units. American Journal of Hospital Palliative Care 2016;33(8):782‐90. [DOI: 10.1177/1049909116630266] - DOI - PubMed
Moore 1998
Moore 2008
-
- Moore RA, Barden J, Derry S, McQuay HJ. Managing potential publication bias. In: McQuay HJ, Kalso E, Moore RA editor(s). Systematic Reviews in Pain Research: Methodology Refined. Seattle: IASP Press, 2008:15‐24. [ISBN: 978–0–931092–69–5]
Moore 2010a
-
- Moore RA, Moore OA, Derry S, Peloso PM, Gammaitoni AR, Wang H. Responder analysis for pain relief and numbers needed to treat in a meta‐analysis of etoricoxib osteoarthritis trials: bridging a gap between clinical trials and clinical practice. Annals of the Rheumatic Diseases 2010;69(2):374‐9. [DOI: 10.1136/ard.2009.107805] - DOI - PMC - PubMed
Moore 2010b
Moore 2013a
Moore 2013b
Moore 2014
Moore 2014a
Moore 2016
Nabal 2012
-
- Nabal M, Librada S, Redondo MJ, Pigni A, Brunelli C, Caraceni A. The role of paracetamol and nonsteroidal anti‐inflammatory drugs in addition to WHO Step III opioids in the control of pain in advanced cancer. A systematic review of the literature. Palliative Medicine 2012;26(4):305‐12. [DOI: 10.1177/0269216311428528] - DOI - PubMed
Nguyen 2017
NICE 2016
-
- National Institute for Health and Care Excellence. Palliative cancer care ‐ pain. cks.nice.org.uk/palliative‐cancer‐care‐pain (accessed 02 February 2017).
Norris 2008
Nüesch 2010
PaPaS 2012
-
- Cochrane Pain, Palliative and Supportive Care Group. PaPaS author and referee guidance. papas.cochrane.org/papas‐documents (accessed 21 November 2016).
PIC 2015
-
- PharmWeb. Paracetamol Information Centre. www2.pharmweb.net/pwmirror/pwy/paracetamol/pharmwebpic.html (accessed 18 January 2016).
Portenoy 1999
Prescott 2000
-
- Prescott LF. Therapeutic misadventure with paracetamol: fact or fiction?. American Journal of Therapeutics 2000;7(2):99‐114. [PUBMED: 11319578] - PubMed
Prommer 2015
-
- Prommer EE. Pharmacological management of cancer‐related pain. Cancer Control 2015;22(4):412‐25. [PUBMED: 26678968] - PubMed
RevMan 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roberts 2015
Roberts 2016
Saragiotto 2016
Schmidt‐Hansen 2015a
Schmidt‐Hansen 2015b
Schwab 2003
Straube 2014
Thorlund 2011
Toms 2008
Turner 2013
Twycross 2014
-
- Twycross R, Wilcock A, Howard P. Palliative Care Formulary. Nottingham: Palliativedrugs.com Ltd, 2014.
Twycross 2015
Van den Beuken‐van Everdingen 2016
Ventafridda 1990
-
- Ventafridda V, Conno F, Panerai AE, Maresca V, Monza GC, Ripamonti C. Non‐steroidal anti‐inflammatory drugs as the first step in cancer pain therapy: double‐blind, within‐patient study comparing nine drugs. Journal of International Medical Research 1990;18(1):21‐9. [DOI: 10.1177/030006059001800104] - DOI - PubMed
WHO 2017
-
- Anonymous. WHO analgesic ladder. www.who.int/cancer/palliative/painladder/en/ (accessed 10 April 2017).
Wiffen 2014
Wiffen 2015
Wiffen 2016
Wiffen 2017
Williams 2014
References to other published versions of this review
McNicol 2015
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical