Correlation of iodine uptake and perfusion parameters between dual-energy CT imaging and first-pass dual-input perfusion CT in lung cancer
- PMID: 28700488
- PMCID: PMC5515760
- DOI: 10.1097/MD.0000000000007479
Correlation of iodine uptake and perfusion parameters between dual-energy CT imaging and first-pass dual-input perfusion CT in lung cancer
Abstract
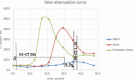
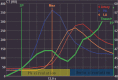
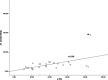
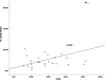
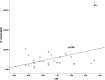
To investigate the potential relationship between perfusion parameters from first-pass dual-input perfusion computed tomography (DI-PCT) and iodine uptake levels estimated from dual-energy CT (DE-CT).The pre-experimental part of this study included a dynamic DE-CT protocol in 15 patients to evaluate peak arterial enhancement of lung cancer based on time-attenuation curves, and the scan time of DE-CT was determined. In the prospective part of the study, 28 lung cancer patients underwent whole-volume perfusion CT and single-source DE-CT using 320-row CT. Pulmonary flow (PF, mL/min/100 mL), aortic flow (AF, mL/min/100 mL), and a perfusion index (PI = PF/[PF + AF]) were automatically generated by in-house commercial software using the dual-input maximum slope method for DI-PCT. For the dual-energy CT data, iodine uptake was estimated by the difference (λ) and the slope (λHU). λ was defined as the difference of CT values between 40 and 70 KeV monochromatic images in lung lesions. λHU was calculated by the following equation: λHU = |λ/(70 - 40)|. The DI-PCT and DE-CT parameters were analyzed by Pearson/Spearman correlation analysis, respectively.All subjects were pathologically proved as lung cancer patients (including 16 squamous cell carcinoma, 8 adenocarcinoma, and 4 small cell lung cancer) by surgery or CT-guided biopsy. Interobserver reproducibility in DI-PCT (PF, AF, PI) and DE-CT (λ, λHU) were relatively good to excellent (intraclass correlation coefficient [ICC]Inter = 0.8726-0.9255, ICCInter = 0.8179-0.8842; ICCInter = 0.8881-0.9177, ICCInter = 0.9820-0.9970, ICCInter = 0.9780-0.9971, respectively). Correlation coefficient between λ and AF, and PF were as follows: 0.589 (P < .01) and 0.383 (P < .05). Correlation coefficient between λHU and AF, and PF were as follows: 0.564 (P < .01) and 0.388 (P < .05).Both the single-source DE-CT and dual-input CT perfusion analysis method can be applied to assess blood supply of lung cancer patients. Preliminary results demonstrated that the iodine uptake relevant parameters derived from DE-CT significantly correlated with perfusion parameters derived from DI-PCT.
Conflict of interest statement
The authors report no conflicts of interest.
Figures


References
-
- Hou WS, Wu HW, Yin Y, et al. Differentiation of lung cancers from inflammatory masses with dual-energy spectral CT imaging. Acad Radiol 2015;22:337–44. - PubMed
-
- Henzler T, Schmid-Bindert G, Fink C. Pulmonary Nodules and Lung Cancer. Berlin Heidelberg: Springer; 2011. 101–9.
-
- Milne EN. Circulation of primary and metastatic pulmonary neoplasms: a postmortem microarteriographic study. Am J Roentgenol Radium TheNucl Med 1967;100:603–19. - PubMed
-
- Yuan X, Zhang J, Quan C, et al. Differentiation of malignant and benign pulmonary nodules with first-pass dual-input perfusion CT. Eur Radiol 2013;23:2469–74. - PubMed
-
- Yuan X, Zhang J, Ao G, et al. Lung cancer perfusion: can we measure pulmonary and bronchial circulation simultaneously? Eur Radiol 2012;22:1665–71. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

